Question: explain step by step solution 3.107 A helium gas is heated at constant volume from a state of 100 kPa, 300 K to 500 K.
explain step by step solution
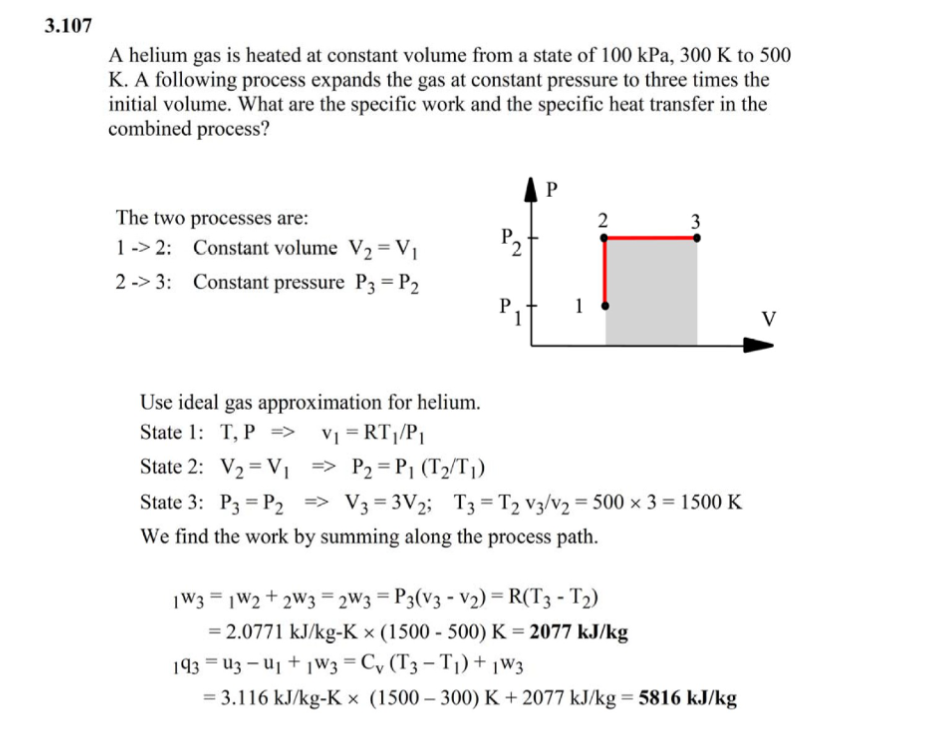 3.107 A helium gas is heated at constant volume from a state of 100 kPa, 300 K to 500 K. A following process expands the gas at constant pressure to three times the initial volume. What are the specific work and the specific heat transfer in the combined process? The two processes are: N 1 -
3.107 A helium gas is heated at constant volume from a state of 100 kPa, 300 K to 500 K. A following process expands the gas at constant pressure to three times the initial volume. What are the specific work and the specific heat transfer in the combined process? The two processes are: N 1 -Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


