Question: Explain these two questions and why is this the answer. 9.BufferAcontains22MMeachofformicacidandsodiumformateandbufferBhas0.4Meachofformicacidand2M sodium formate. Which solution will have the higher buffer capacity? (formic is a weak
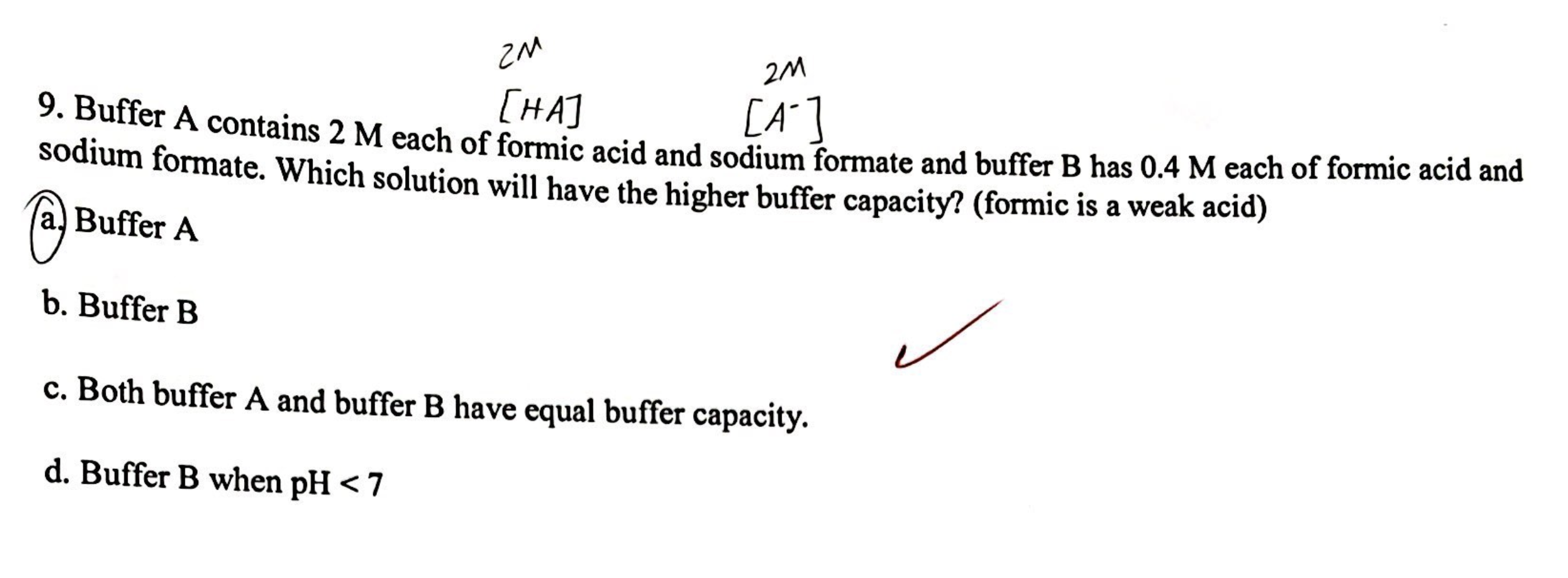
 Explain these two questions and why is this the answer.
Explain these two questions and why is this the answer.
9.BufferAcontains22MMeachofformicacidandsodiumformateandbufferBhas0.4Meachofformicacidand2M sodium formate. Which solution will have the higher buffer capacity? (formic is a weak acid) (a.) Buffer A b. Buffer B c. Both buffer A and buffer B have equal buffer capacity. d. Buffer B when pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


