Question: explain why 7. Consider the two statements below. Which of the following best explains (I) and (II)? (I) the Ka of HXO2 is greater than
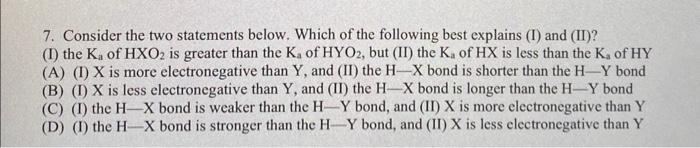
7. Consider the two statements below. Which of the following best explains (I) and (II)? (I) the Ka of HXO2 is greater than the Ka of HYO2, but (II) the Ka of HX is less than the Ka of HY (A) (I) X is more electronegative than Y, and (II) the HX bond is shorter than the HY bond (B) (I) X is less electronegative than Y, and (II) the HX bond is longer than the HY bond (C) (I) the HX bond is weaker than the HY bond, and (II) X is more electronegative than Y (D) (I) the HX bond is stronger than the HY bond, and (II) X is less electronegative than Y
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


