Question: Explain why hydrogen fluoride (HF) has a higher boiling temperature than hydrogen chloride (HCl)(19.4C vs. 85C), even though HF has a lower molecular weight. The
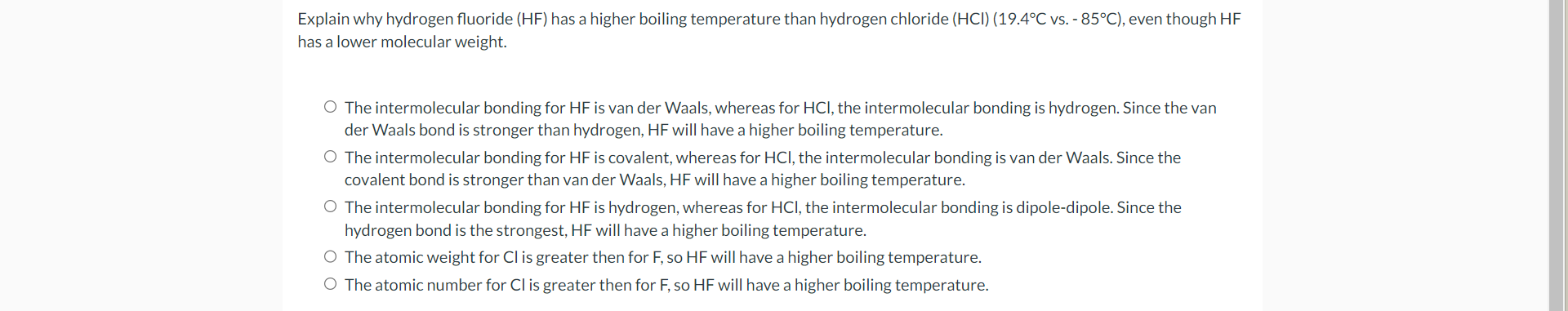
Explain why hydrogen fluoride (HF) has a higher boiling temperature than hydrogen chloride (HCl)(19.4C vs. 85C), even though HF has a lower molecular weight. The intermolecular bonding for HF is van der Waals, whereas for HCl, the intermolecular bonding is hydrogen. Since the van der Waals bond is stronger than hydrogen, HF will have a higher boiling temperature. The intermolecular bonding for HF is covalent, whereas for HCl, the intermolecular bonding is van der Waals. Since the covalent bond is stronger than van der Waals, HF will have a higher boiling temperature. The intermolecular bonding for HF is hydrogen, whereas for HCl, the intermolecular bonding is dipole-dipole. Since the hydrogen bond is the strongest, HF will have a higher boiling temperature. The atomic weight for Cl is greater then for F, so HF will have a higher boiling temperature. The atomic number for Cl is greater then for F, so HF will have a higher boiling temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


