Question: Explain why II is not the answer if it can act both based on my solution. Please also check my solution. Thanks. 3. Which of
Explain why II is not the answer if it can act both based on my solution. Please also check my solution. Thanks.
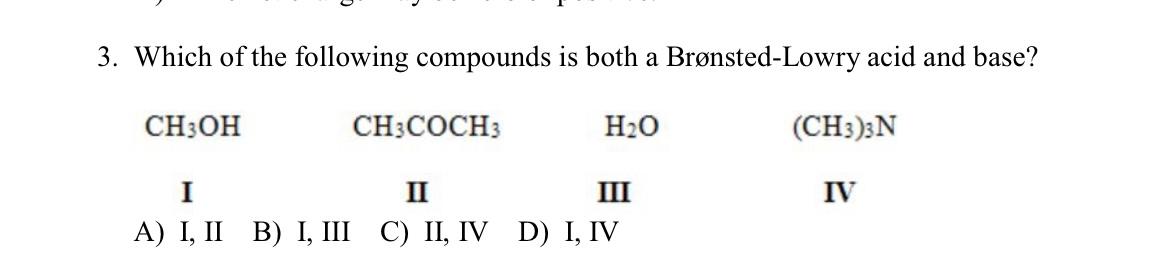
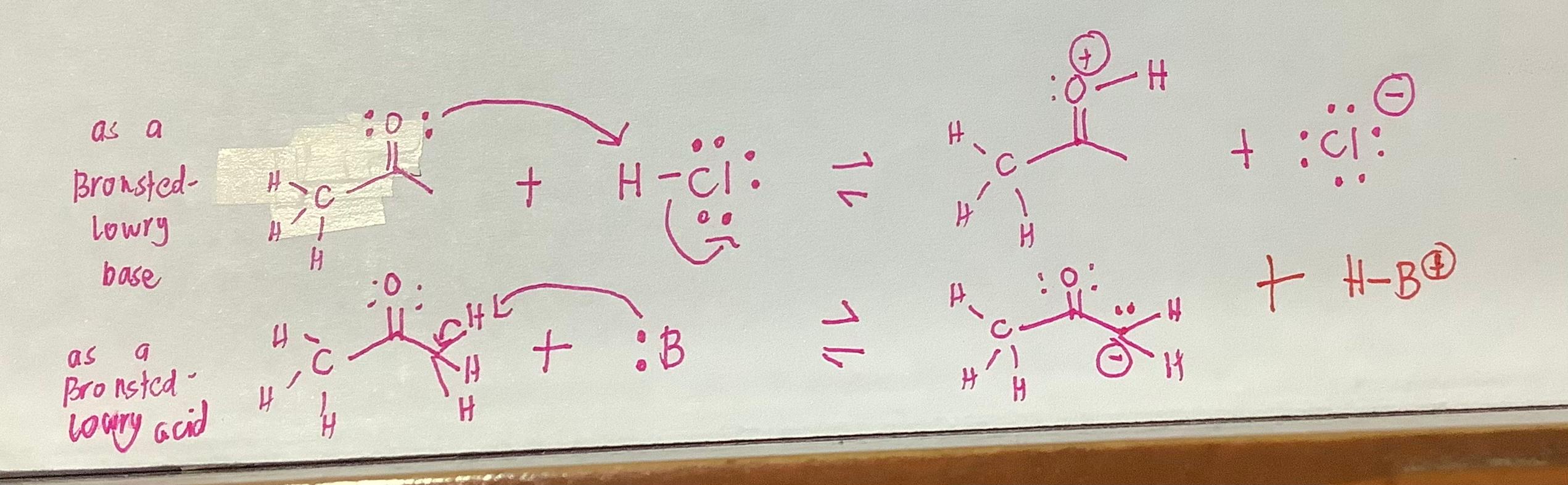
3. Which of the following compounds is both a Brnsted-Lowry acid and base? CH3OH CH3COCH H20 (CH3)3N IV I II MI A) I, II B) I, III C) II, IV D) I, IV H as a + C t :cl: + -C H-ci: + 1 Bronsted- Lowry base ? 41 H M 0. . t # + H H-80 H CHE a 11 At :B # as Bronsted towry acid - 14 H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


