Question: Explain your reasoning. Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help molar mass CH3CH3
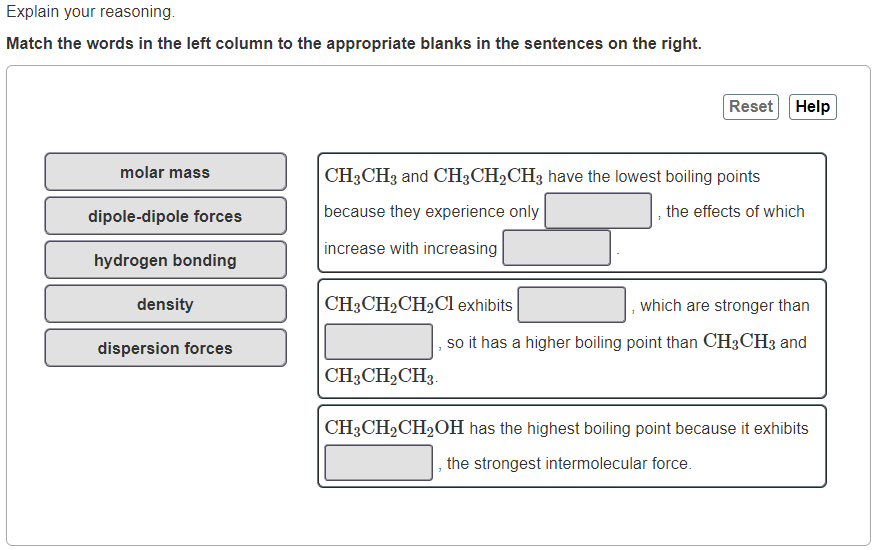
Explain your reasoning. Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help molar mass CH3CH3 and CH3CH2CH3 have the lowest boiling points because they experience only the effects of which dipole-dipole forces increase with increasing hydrogen bonding density dispersion forces CH3CH2CH2Cl exhibits which are stronger than so it has a higher boiling point than CH3CH3 and CH3CH2CH3 CH3CH2CH2OH has the highest boiling point because it exhibits the strongest intermolecular force
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


