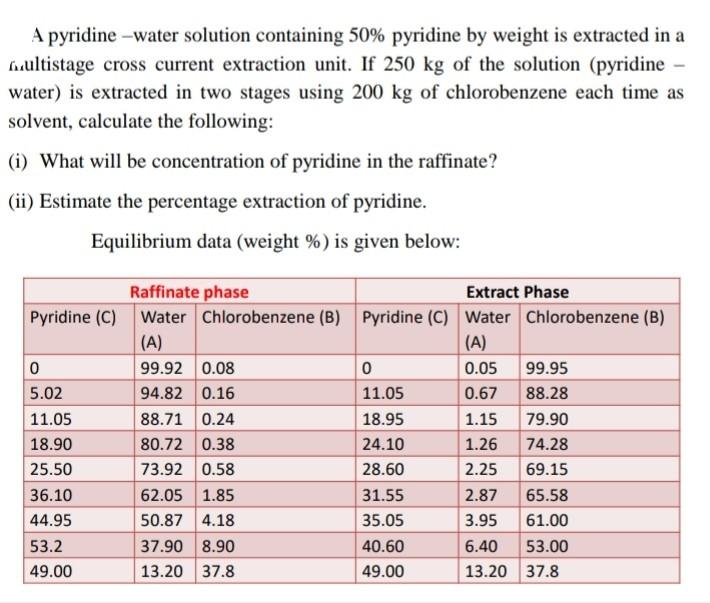
Question: Explained properly with Graph...and mentioned all steps Correct solution needed here and don't copy from internet sources. A pyridine -water solution containing 50% pyridine by
Explained properly with Graph...and mentioned all steps Correct solution needed here and don't copy from internet sources.
A pyridine -water solution containing 50% pyridine by weight is extracted in a fuultistage cross current extraction unit. If 250kg of the solution (pyridine water) is extracted in two stages using 200kg of chlorobenzene each time as solvent, calculate the following: (i) What will be concentration of pyridine in the raffinate? (ii) Estimate the percentage extraction of pyridine. Equilibrium data (weight % ) is given below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


