Question: explained step by step please The following cell was built Pt|H2(g)(43,5 kPa) see statement||see statement|Ag(s) For the electrode on the right, 25.00 mL of NagP04
explained step by step please
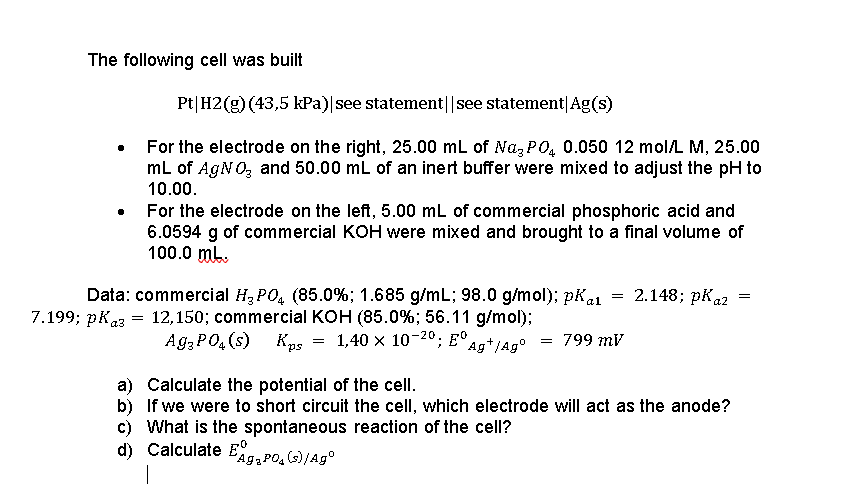
The following cell was built Pt|H2(g)(43,5 kPa) see statement||see statement|Ag(s) For the electrode on the right, 25.00 mL of NagP04 0.050 12 mol/L M, 25.00 mL of AgNO3 and 50.00 mL of an inert buffer were mixed to adjust the pH to 10.00. For the electrode on the left, 5.00 mL of commercial phosphoric acid and 6.0594 g of commercial KOH were mixed and brought to a final volume of 100.0 mb. Data: commercial H3PO4 (85.0%; 1.685 g/mL; 98.0 g/mol); pKai = 2.148; pK a2 7.199; PK23 = 12,150; commercial KOH (85.0%; 56.11 g/mol); Ag3PO4(s) 1,40 x 10-20; E Agt/Ag = 799 mV = Kps a) Calculate the potential of the cell. b) If we were to short circuit the cell, which electrode will act as the anode? c) What is the spontaneous reaction of the cell? d) Calculate E EAG,PO.()/Ag s/ The following cell was built Pt|H2(g)(43,5 kPa) see statement||see statement|Ag(s) For the electrode on the right, 25.00 mL of NagP04 0.050 12 mol/L M, 25.00 mL of AgNO3 and 50.00 mL of an inert buffer were mixed to adjust the pH to 10.00. For the electrode on the left, 5.00 mL of commercial phosphoric acid and 6.0594 g of commercial KOH were mixed and brought to a final volume of 100.0 mb. Data: commercial H3PO4 (85.0%; 1.685 g/mL; 98.0 g/mol); pKai = 2.148; pK a2 7.199; PK23 = 12,150; commercial KOH (85.0%; 56.11 g/mol); Ag3PO4(s) 1,40 x 10-20; E Agt/Ag = 799 mV = Kps a) Calculate the potential of the cell. b) If we were to short circuit the cell, which electrode will act as the anode? c) What is the spontaneous reaction of the cell? d) Calculate E EAG,PO.()/Ag s/
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


