Question: explained step by step please The titration of 25.00 mL of a solution of tin (II) 0.0200 mol/L in hydrochloric acid 1.00 mol/L, with vanadium
explained step by step please
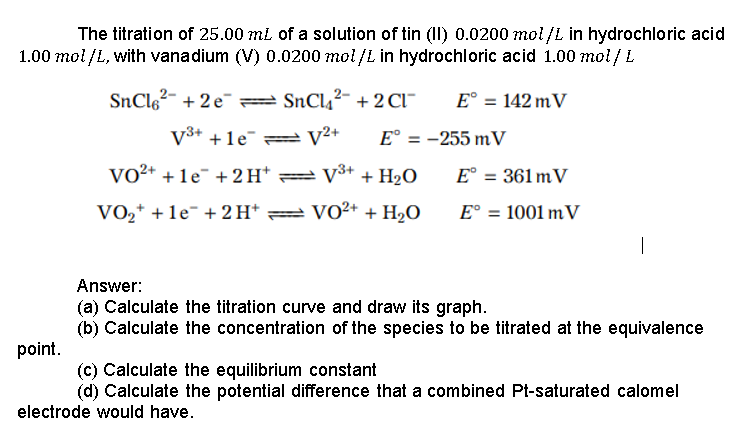
The titration of 25.00 mL of a solution of tin (II) 0.0200 mol/L in hydrochloric acid 1.00 mol/L, with vanadium (V) 0.0200 mol/L in hydrochloric acid 1.00 mol/L SnC162- +2e=SnCl2- + 2 C1" E = 142 mV V3+ +1e = V2+ E = -255 m V VO2+ + 1e" + 2H=V3+ + H2O E = 361 m V VO2+ +1e- + 2H+ = VO2+ + H2O E = 1001 mV | Answer: (a) Calculate the titration curve and draw its graph. (b) Calculate the concentration of the species to be titrated at the equivalence point. (c) Calculate the equilibrium constant (d) Calculate the potential difference that a combined Pt-saturated calomel electrode would have
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


