Question: Explanation #2: The C-O bond at cm involves an sp2 hybridized carbon atom, rather than an sp hybridized carbon atom. A Cisp)- O has s-character


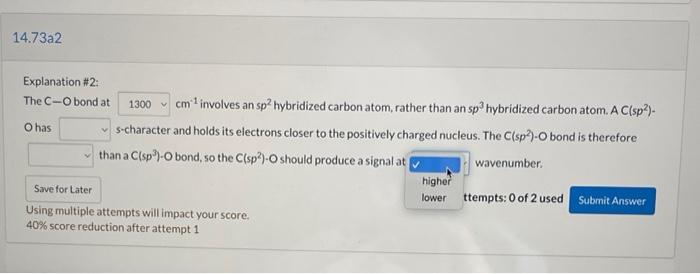

Explanation #2: The C-O bond at cm involves an sp2 hybridized carbon atom, rather than an sp hybridized carbon atom. A Cisp)- O has s-character and holds its electrons closer to the positively charged nucleus. The Clsp?)-O bond is therefore than a Clsp)-O bond, so the Clsp)-Oshould produce a signal at , wavenumber. Attempts: 0 of 2 used Submit Answer Save for Later Using multiple attempts will impact your score. 40% score reduction after attempt 1 14.73a2 Explanation #2: The C-O bond at 1300 cm involves an sp2 hybridized carbon atom, rather than an sp hybridized carbon atom. A C(sp? O has s-character and holds its electrons closer to the positively charged nucleus. The Clsp)-O bond is therefore less han a C(sp)-O bond, so the C(sp)-O should produce a signal at wavenumber. more Attempts: 0 of 2 used Submit Answer Save for Later Using multiple attempts will impact your score. 40% score reduction after attempt 1 14.73a2 1300 O has Explanation #2: The C-Obond at cm involves an sp2 hybridized carbon atom, rather than an sp hybridized carbon atom A Clsp)- s-character and holds its electrons closer to the positively charged nucleus. The Clsp?)-O bond is therefore than a Clsp)-O bond, so the C(sp) should produce a signal at wavenumber higher lower ttempts: 0 of 2 used Submit Answer Using multiple attempts will impact your score. 40% score reduction after attempt 1 Save for Later 14.73a2 Explanation #2: The C-O bond at 1300cm involves an sp hybridized carbon atom, rather than an sp hybridized carbon atom. A C(sp?). O has s-character and holds its electrons closer to the positively charged nucleus. The C(sp)-O bond is therefore than a Clsp)-O bond, so the C(sp)-Oshould produce a signal at wavenumber. stronger weaker Attempts: 0 of 2 used Submit Answer Using multiple attempts will impact your score. 40% score reduction after attempt 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


