Question: explanation using key words and concepts under the question Based on the vapo-pressure curve for (CH3)3COH, which is its normal boiling point? Keywords: normal boiling
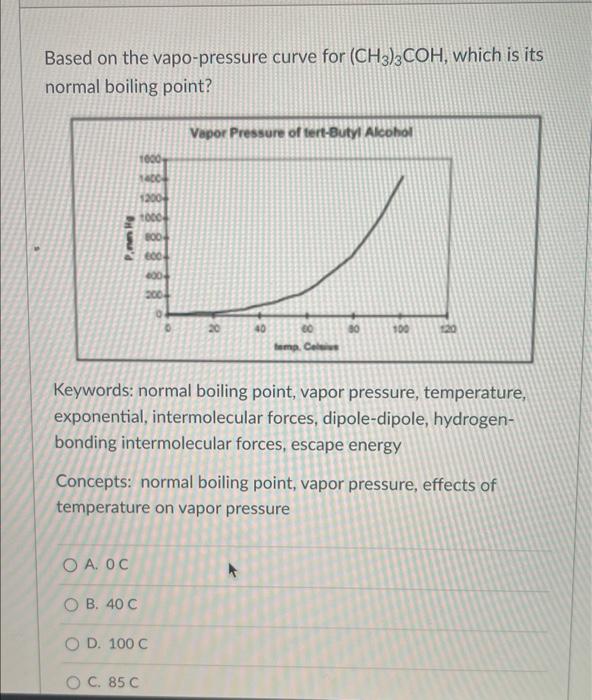
Based on the vapo-pressure curve for (CH3)3COH, which is its normal boiling point? Keywords: normal boiling point, vapor pressure, temperature, exponential, intermolecular forces, dipole-dipole, hydrogenbonding intermolecular forces, escape energy Concepts: normal boiling point, vapor pressure, effects of temperature on vapor pressure A. 0C B. 40C D. 100C C. 85C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


