Question: explanation using keywords and concepts stated under the question. Which compound demonstrates only dipole-dipole interactions? Keywords: dipole moment, polarity, electronegativity, partial negative, partial positive, Coulombic
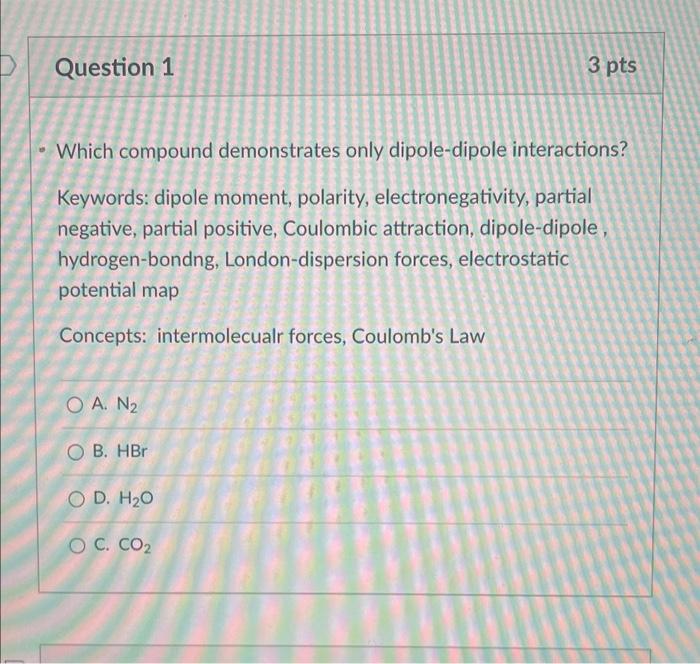
Which compound demonstrates only dipole-dipole interactions? Keywords: dipole moment, polarity, electronegativity, partial negative, partial positive, Coulombic attraction, dipole-dipole, hydrogen-bondng, London-dispersion forces, electrostatic potential map Concepts: intermolecualr forces, Coulomb's Law A. N2 B. HBr D. H2O C. CO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


