Question: (f) Using the data in the table, determine the value of the equilibrium constant, Kp, for the reaction represented by the equation below. Cl2(g)+CO(g)COCl2(g) (g)
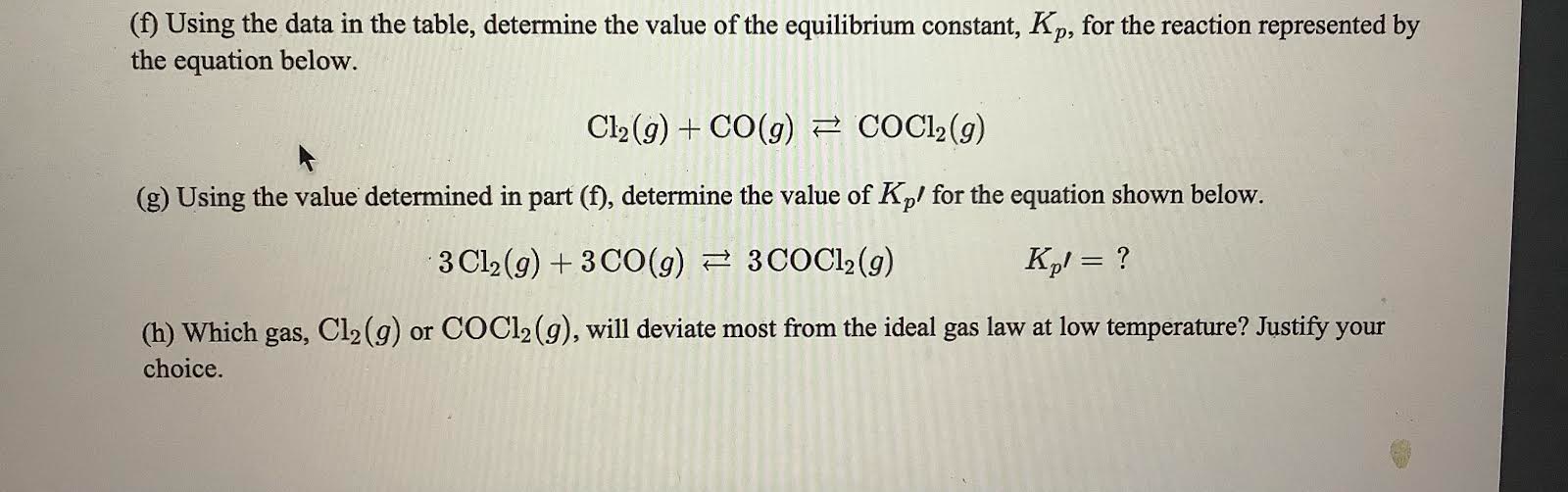
(f) Using the data in the table, determine the value of the equilibrium constant, Kp, for the reaction represented by the equation below. Cl2(g)+CO(g)COCl2(g) (g) Using the value determined in part (f), determine the value of Kp ' for the equation shown below. 3Cl2(g)+3CO(g)3COCl2(g)Kp=? (h) Which gas, Cl2(g) or COCl2(g), will deviate most from the ideal gas law at low temperature? Justify your choice. (f) Using the data in the table, determine the value of the equilibrium constant, Kp, for the reaction represented by the equation below. Cl2(g)+CO(g)COCl2(g) (g) Using the value determined in part (f), determine the value of Kp ' for the equation shown below. 3Cl2(g)+3CO(g)3COCl2(g)Kp=? (h) Which gas, Cl2(g) or COCl2(g), will deviate most from the ideal gas law at low temperature? Justify your choice
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


