Question: Write the condensed formula for dichloromethane. 3. a. Construct a model of ethane with two carbons connected together and the remaining six posi- tions with
Write the condensed formula for dichloromethane. 3.
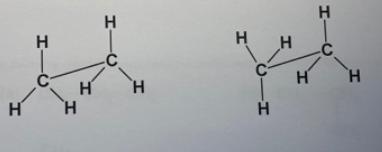
a. Construct a model of ethane with two carbons connected together and the remaining six posi- tions with hydrogens. Draw the full structural formula for ethane.
b. Notice that you can twist the model of ethane around the carbon carbon bond so that the hydrogens on one carbon rotate successively into positions opposite each of the hydro gens on the other carbon. This is called free rotation about a single covalent bond or conformational rotation and occurs within molecules very rapidly at room temperature. Three-dimensional pictures of the different rotational positions your model can make are called conformations. Conformations do not represent different molecules since free rotation is possible. Do these pictures represent the same compound or different ones?
H H. H. H O
Step by Step Solution
3.57 Rating (157 Votes )
There are 3 Steps involved in it
The conden... View full answer

Get step-by-step solutions from verified subject matter experts


