Question: fA. Determine which base, propoxide or propionate, would be more effective in completely deprotonating ethanethiol. Draw your chosen base and its conjugate acid in the
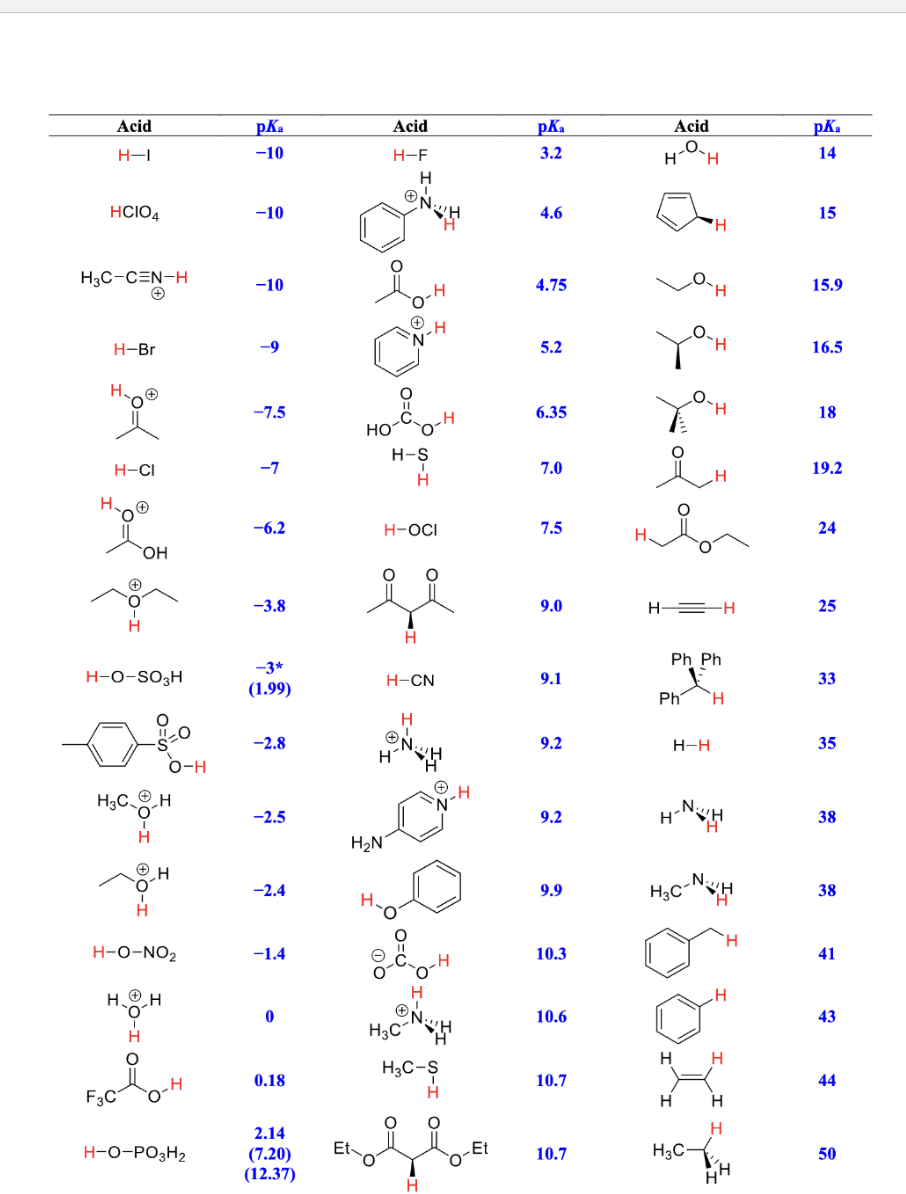
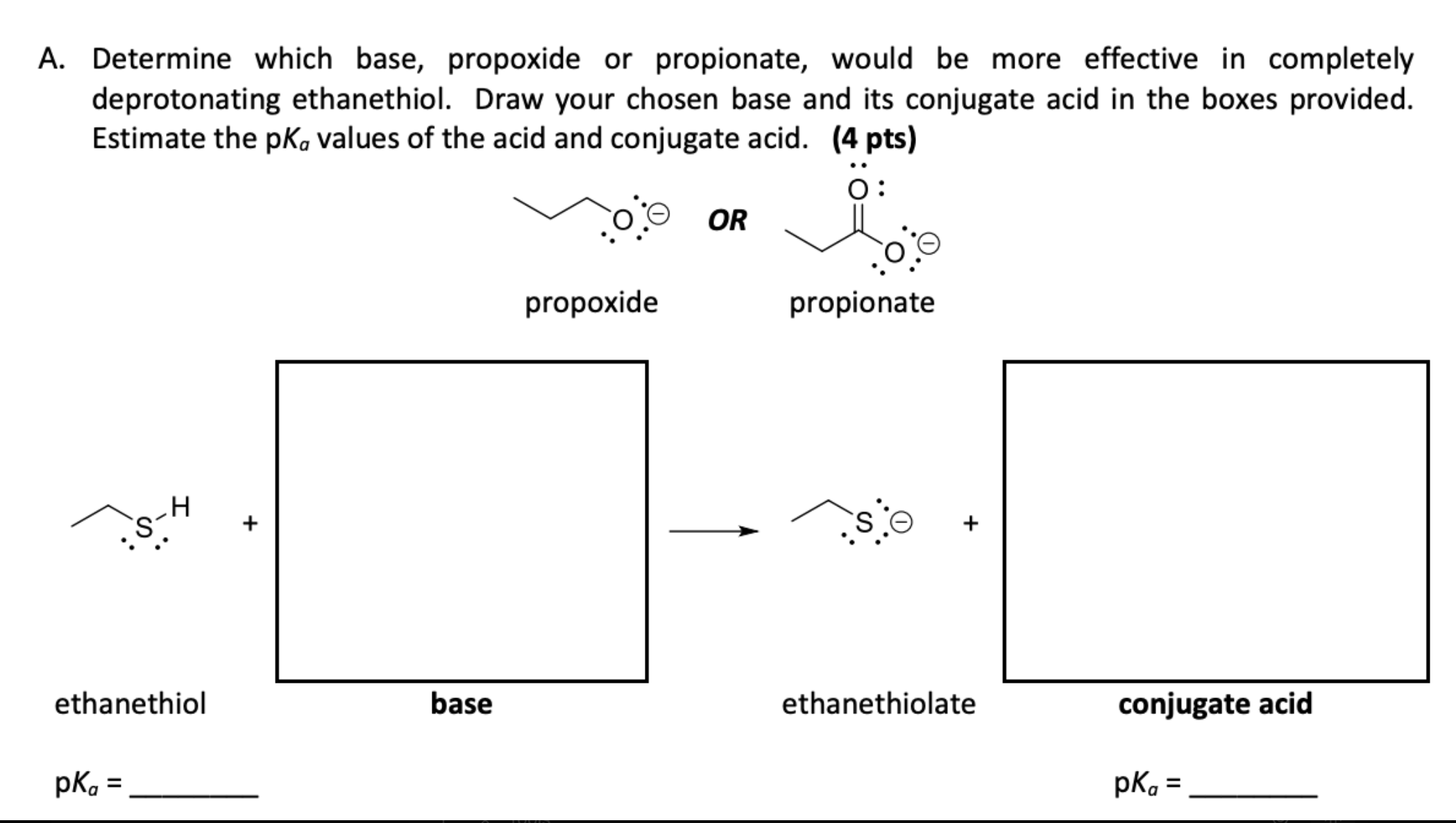 \fA. Determine which base, propoxide or propionate, would be more effective in completely deprotonating ethanethiol. Draw your chosen base and its conjugate acid in the boxes provided. Estimate the pka values of the acid and conjugate acid. (4 pts) O : OR propoxide propionate - H S + + ethanethiol base ethanethiolate conjugate acid pka = pka =
\fA. Determine which base, propoxide or propionate, would be more effective in completely deprotonating ethanethiol. Draw your chosen base and its conjugate acid in the boxes provided. Estimate the pka values of the acid and conjugate acid. (4 pts) O : OR propoxide propionate - H S + + ethanethiol base ethanethiolate conjugate acid pka = pka =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


