Question: fast .. A +X = A-X forward: k, reverse: k-1 B + X = B-X forward: k2, reverse: k-2 A-X + B-X P-X + X

fast ..
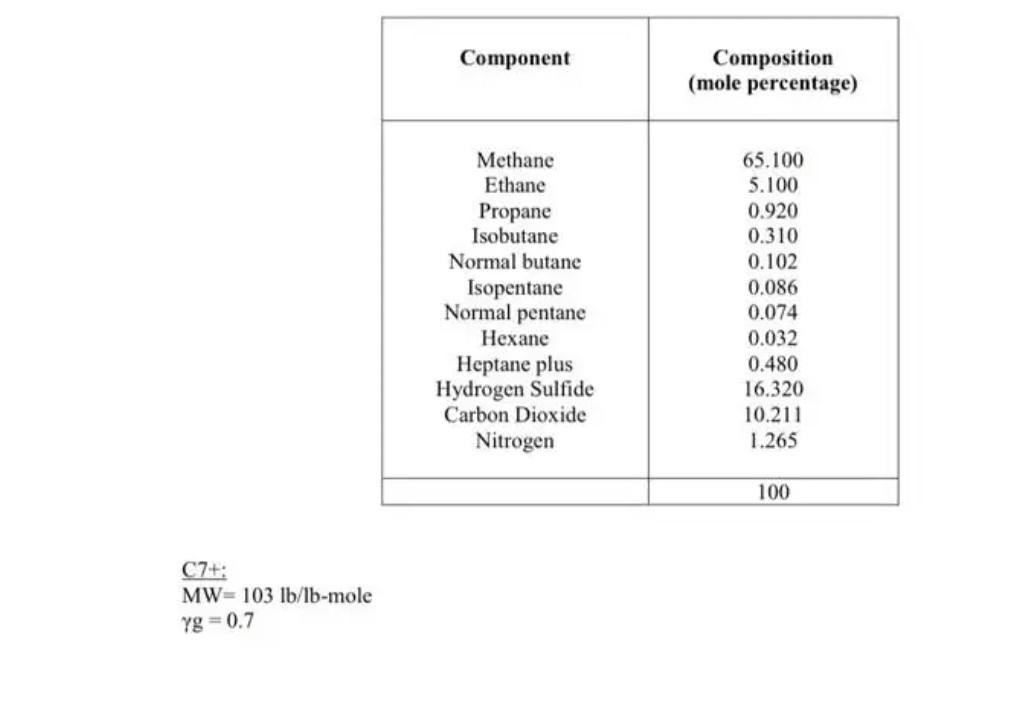
A +X = A-X forward: k, reverse: k-1 B + X = B-X forward: k2, reverse: k-2 A-X + B-X P-X + X kz P+X =P - X forward: k, reverse: k-4 At 373 K, the equilibrium constants for adsorption are KE = 190,000 K2 = 580,000 K4 75,000 cm /mol The heats of adsorption are , = -20,000 AH2 = -30,000 , = -12,000 cal/mol Thorato co the Worcibo co ronction is Component Composition (mole percentage) Methane Ethane Propane Isobutane Normal butane Isopentane Normal pentane Hexane Heptane plus Hydrogen Sulfide Carbon Dioxide Nitrogen 65.100 5.100 0.920 0.310 0.102 0.086 0.074 0.032 0.480 16.320 10.211 1.265 100 C7+: MW= 103 lb/Ib-mole Yg=0.7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


