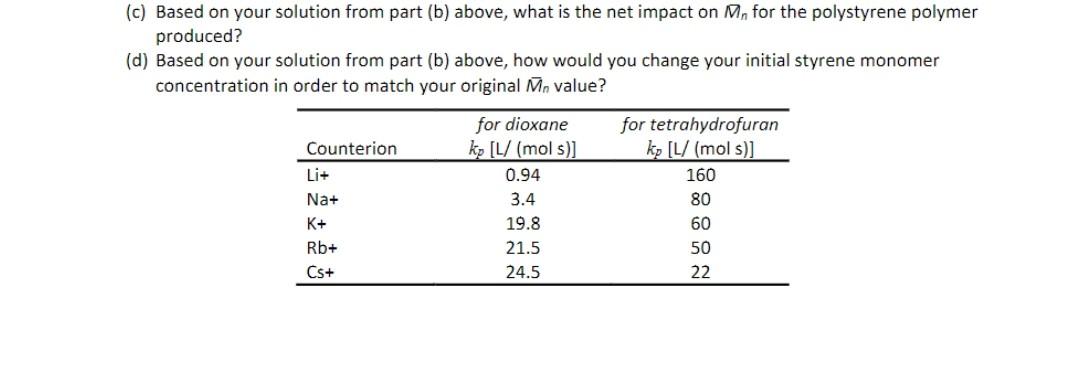
Question: fast.. (c) Based on your solution from part (b) above, what is the net impact on M, for the polystyrene polymer produced? (d) Based on
fast..
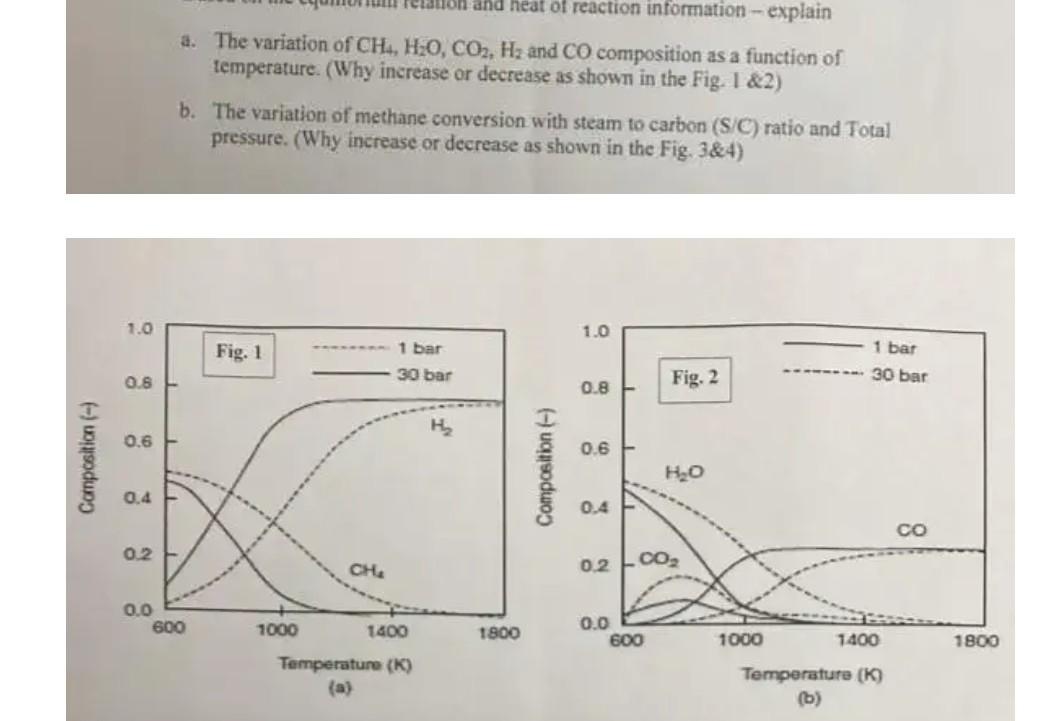
(c) Based on your solution from part (b) above, what is the net impact on M, for the polystyrene polymer produced? (d) Based on your solution from part (b) above, how would you change your initial styrene monomer concentration in order to match your original Mn value? for dioxane for tetrahydrofuran Counterion ko [L/ (mol s)] kp [L/ (mol s)] 0.94 160 Na+ 3.4 80 K+ 19.8 60 Rb+ 21.5 50 Cs+ 24.5 22 and heat of reaction information - explain a. The variation of CH., H2O, CO2, Hz and CO composition as a function of temperature. (Why increase or decrease as shown in the Fig. 1&2) b. The variation of methane conversion with steam to carbon (S/C) ratio and Total pressure. (Why increase or decrease as shown in the Fig. 3&4) 1.0 1.0 Fig. 1 1 bar 1 bar 30 bar 0.6 30 bar 0.8 Fig. 2 H 0.6 0.6 Composition (-) Composition ) HO 0.4 0.4 CO 02 CHA 02 co, 0.0 600 1000 1400 1800 0.0 600 1000 1400 1800 Temperature (K) (a) Temperature (K) (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


