Question: Fe - 10 atom % C austenite (fcc), having no Fe vacancies, parameter of 4 . The density of austenite in g cm is
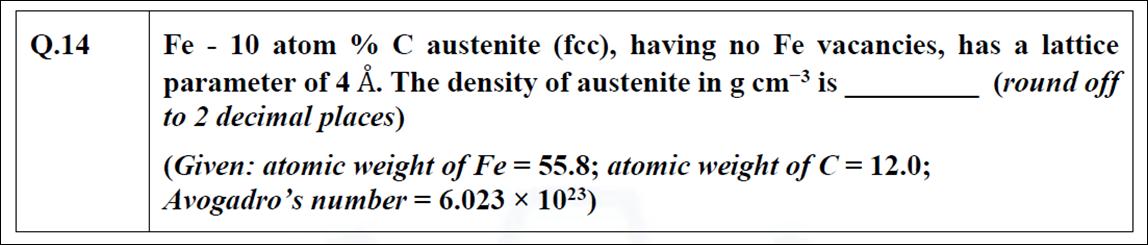
Fe - 10 atom % C austenite (fcc), having no Fe vacancies, parameter of 4 . The density of austenite in g cm is to 2 decimal places) (Given: atomic weight of Fe = 55.8; atomic weight of C = 12.0; Avogadro's number = 6.023 10) has a lattice (round off
Step by Step Solution
There are 3 Steps involved in it
The detailed an... View full answer

Get step-by-step solutions from verified subject matter experts


