Question: Feed = 1 0 0 0 k g ; A benzene ) = ( 7 8 nitrobenzenc ( M M 1 1 2 3 )
Feed ; benzene nitrobenzenc mol. wt of feed
Moles of beed, kmol.
Residue in the still kmol, mole fraction of beuzene ; mass of residue
Use Eq put the values of and
Solvig, Use Eq formatesial balance
The repossed concentration of benzene iu iu the accumulated condensate is not accirate.
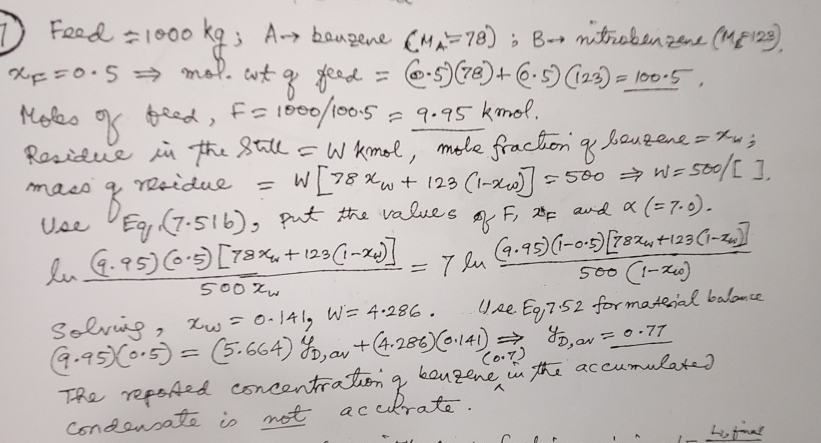
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


