Question: FEEDBACK TO Help with questions ^^^ ANSWER!! Q3): From graph, Rmin=0.75; xw=0.033; 8 theoretical plates plus the reboiler; feed on stage 3, Some key points
FEEDBACK TO Help with questions ^^^
ANSWER!! Q3): From graph, Rmin=0.75; xw=0.033; 8 theoretical plates plus the reboiler; feed on stage 3, 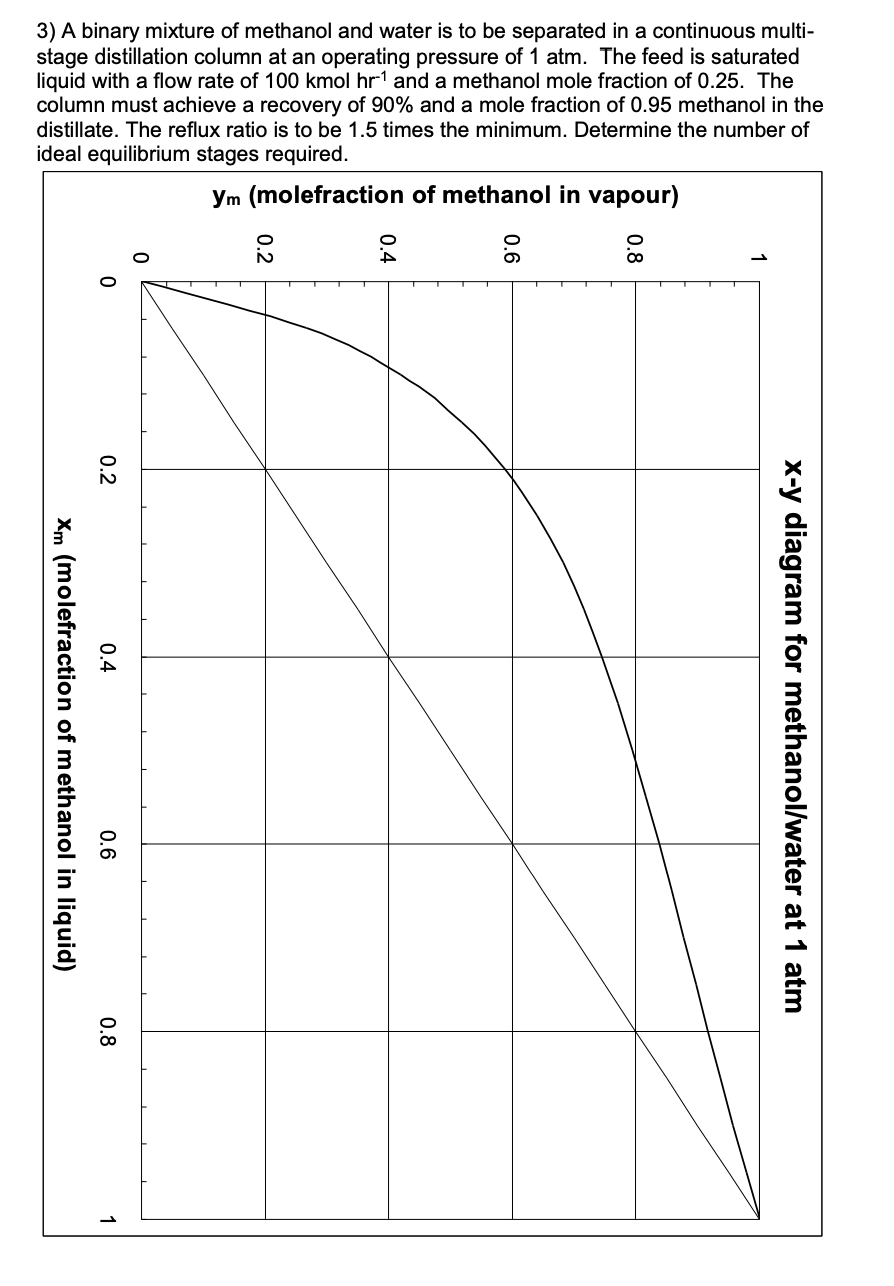
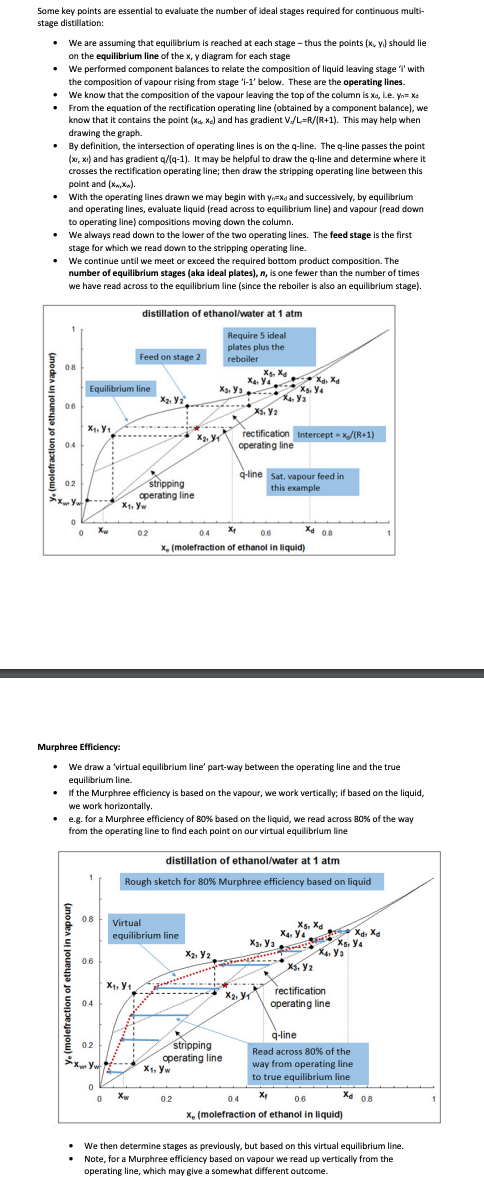
Some key points are essential to evaluate the number of ideal stages required for continuous multistage distillation: - We are assuming that equilibrium is reached at each stage -thus the points (x4,yi) should lie on the equilibrium line of the x, y diagram for each stage - We performed component balances to relate the composition of liquid leaving stage 'i' with the composition of vapour rising from stage ' i1 ' below. These are the operating lines. - We know that the composition of the vapour leaving the top of the column is xd, L.e. yn=xa - From the equation of the rectification operating line (obtained by a component balance), we know that it contains the point (xd,x) and has gradient Vd/L=R/(R+1). This may help when drawing the graph. - By definition, the intersection of operating lines is on the q-line. The q-line passes the point (xr,x1) and has gradient q/(q1). It may be helpful to draw the q-line and determine where it crosses the rectification operating line; then draw the stripping operating line between this point and (xN,xn). - With the operating lines drawn we may begin with yn=xd and successively, by equilibrium and operating lines, evaluate liquid (read across to equilibrium line) and vapour (read down to operating line) compositions moving down the column. - We always read down to the lower of the two operating lines. The feed stage is the first stage for which we read down to the stripping operating line. - We continue until we meet or exceed the required bottom product composition. The number of equilibrium stages (aka ideal plates), n, is one fewer than the number of times we have read across to the equilibrium line (since the reboiler is also an equilibrium stage). Murphree Efficiency: - We draw a 'virtual equilibrium line' part-way between the operating line and the true equilibrium line. - If the Murphree efficiency is based on the vapour, we work vertically; if based on the liquid, we work horizontally. - e.g. for a Murphree efficiency of 80% based on the liquid, we read across 80% of the way from the operating line to find each point on our virtual equilibrium line - We then determine stages as previously, but based on this virtual equilibrium line. - Note, for a Murphree efficiency based on vapour we read up vertically from the operating line, which may give a somewhat different outcome. 3) A binary mixture of methanol and water is to be separated in a continuous multistage distillation column at an operating pressure of 1atm. The feed is saturated liquid with a flow rate of 100kmolhr1 and a methanol mole fraction of 0.25 . The column must achieve a recovery of 90% and a mole fraction of 0.95 methanol in the distillate. The reflux ratio is to be 1.5 times the minimum. Determine the number of ideal equilibrium stages required
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


