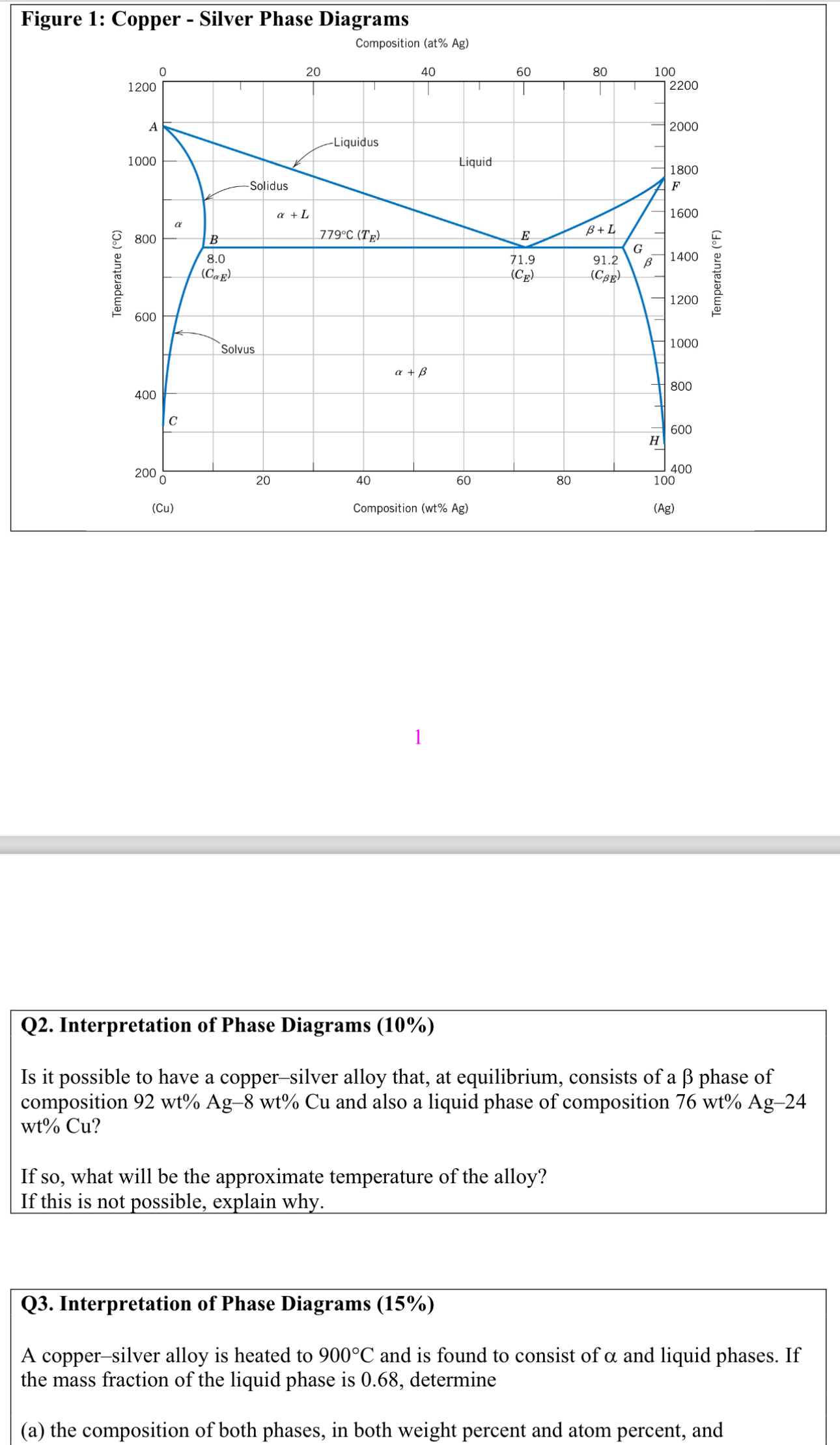
Question: Figure 1 : Copper - Silver Phase Diagrams Composition ( at % Ag ) ( Cu ) Composition ( wt % Ag ) ( Ag
Figure : Copper Silver Phase Diagrams
Composition at Ag
Cu
Composition wt Ag
Ag
Q Interpretation of Phase Diagrams
Is it possible to have a coppersilver alloy that, at equilibrium, consists of a phase of composition and also a liquid phase of composition
If so what will be the approximate temperature of the alloy?
If this is not possible, explain why.
Q Interpretation of Phase Diagrams
A coppersilver alloy is heated to and is found to consist of and liquid phases. If the mass fraction of the liquid phase is determine
a the composition of both phases, in both weight percent and atom percent, and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


