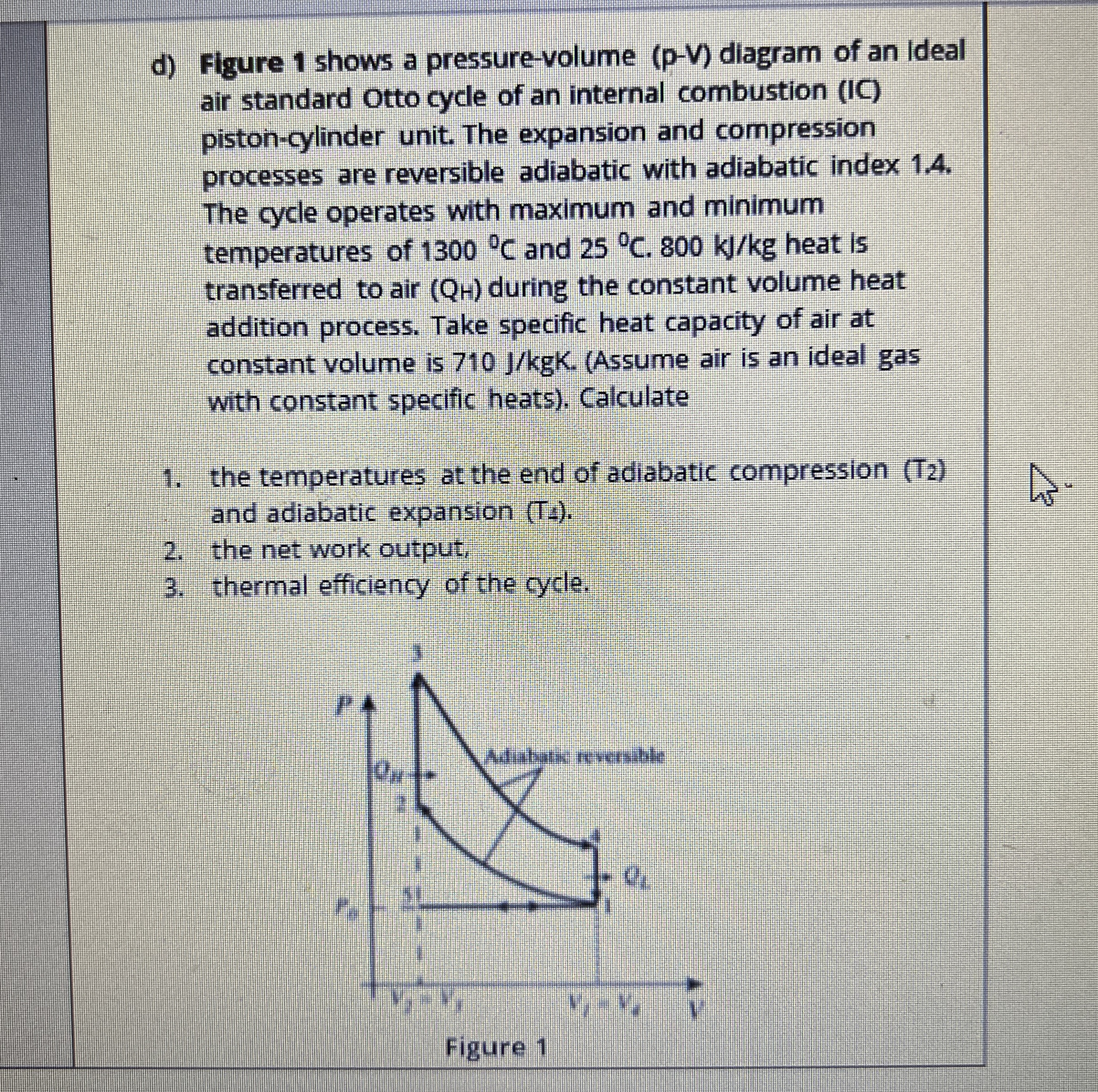
Question: Figure 1 shows a pressure - volume ( p - V ) diagram of an Ideal air standard Otto cycle of an internal combustion (
Figure shows a pressurevolume pV diagram of an Ideal air standard Otto cycle of an internal combustion IC pistoncylinder unit. The expansion and compression processes are reversible adiabatic with adiabatic index The cycle operates with maximum and minimum temperatures of deg C and deg C kJkg heat is transferred to air Q during the constant volume heat addition process. Take specific heat capacity of air at constant volume is JkgKAssume air is an ideal gas with constant specific heats Calculatethe temperatures at the end of adiabatic compression T and adiabatic expansion Tathe net work output, thermal efficiency of the cycle.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


