Question: Figure 1 shows the equilibrium miscibility data for the system acetic acid-water-isopropyl ether at 101 kPa and 20C. We have a feed of 15 kg
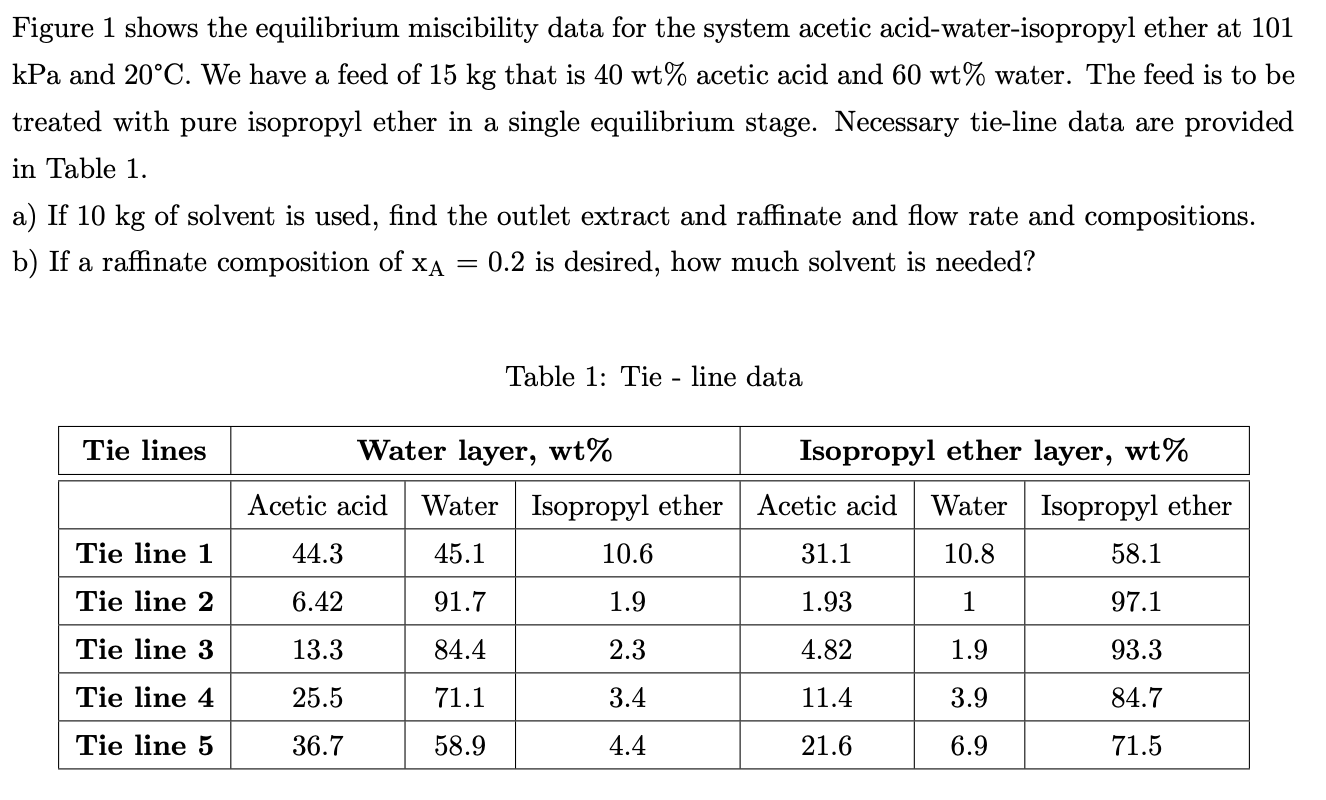
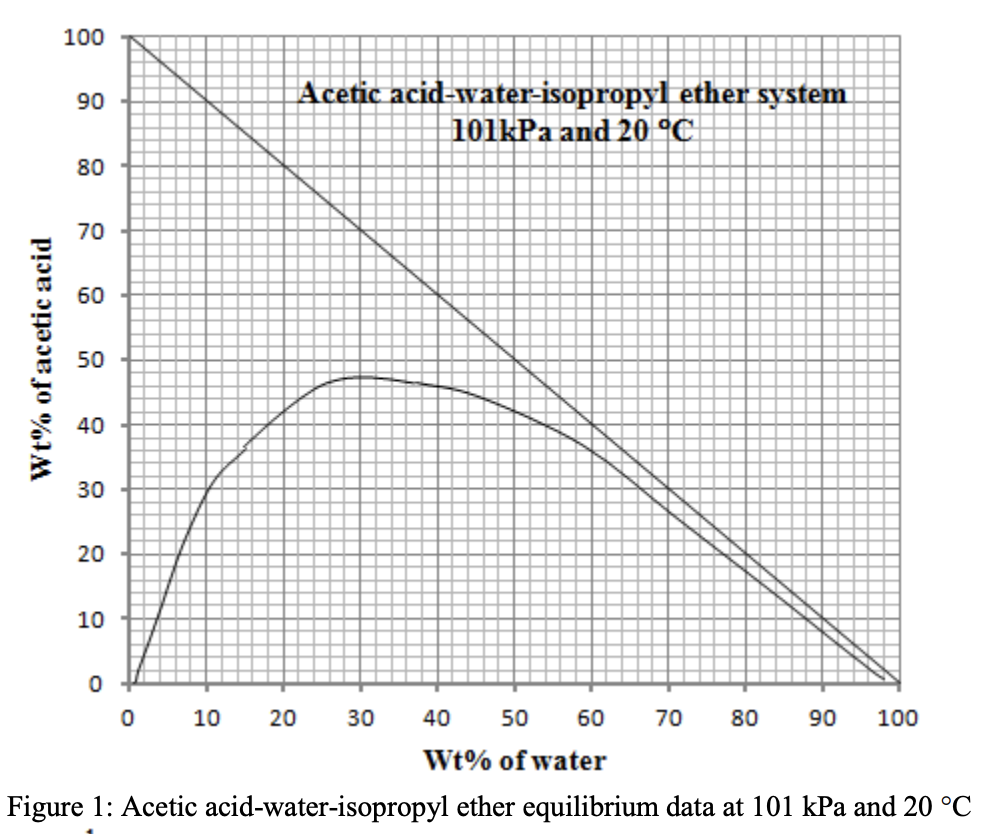
Figure 1 shows the equilibrium miscibility data for the system acetic acid-water-isopropyl ether at 101 kPa and 20C. We have a feed of 15 kg that is 40 wt% acetic acid and 60 wt% water. The feed is to be treated with pure isopropyl ether in a single equilibrium stage. Necessary tie-line data are provided in Table 1. a) If 10 kg of solvent is used, find the outlet extract and raffinate and flow rate and compositions. b) If a raffinate composition of XA 0.2 is desired, how much solvent is needed? = Table 1: Tie - line data Tie lines Isopropyl ether layer, wt% Water layer, wt% Acetic acid Water Isopropyl ether 44.3 45.1 10.6 Acetic acid Water Isopropyl ether 10.8 58.1 Tie line 1 31.1 Tie line 2 6.42 91.7 1.9 1.93 1 97.1 Tie line 3 13.3 84.4 2.3 4.82 1.9 93.3 Tie line 4 25.5 71.1 3.4 11.4 3.9 84.7 Tie line 5 36.7 58.9 4.4 21.6 6.9 71.5 100 90 Acetic acid-water-isopropyl ether system 101kPa and 20 C 80 70 60 Wt% of acetic acid 50 40 30 20 10 0 0 10 20 30 40 50 60 70 80 90 100 Wt% of water Figure 1: Acetic acid-water-isopropyl ether equilibrium data at 101 kPa and 20 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


