Question: Fill in the blanks by selecting one option from each menu. 1,000,000 ab Part 1: 2 points 100,000 aby Part 2: 2 points Solid SCF
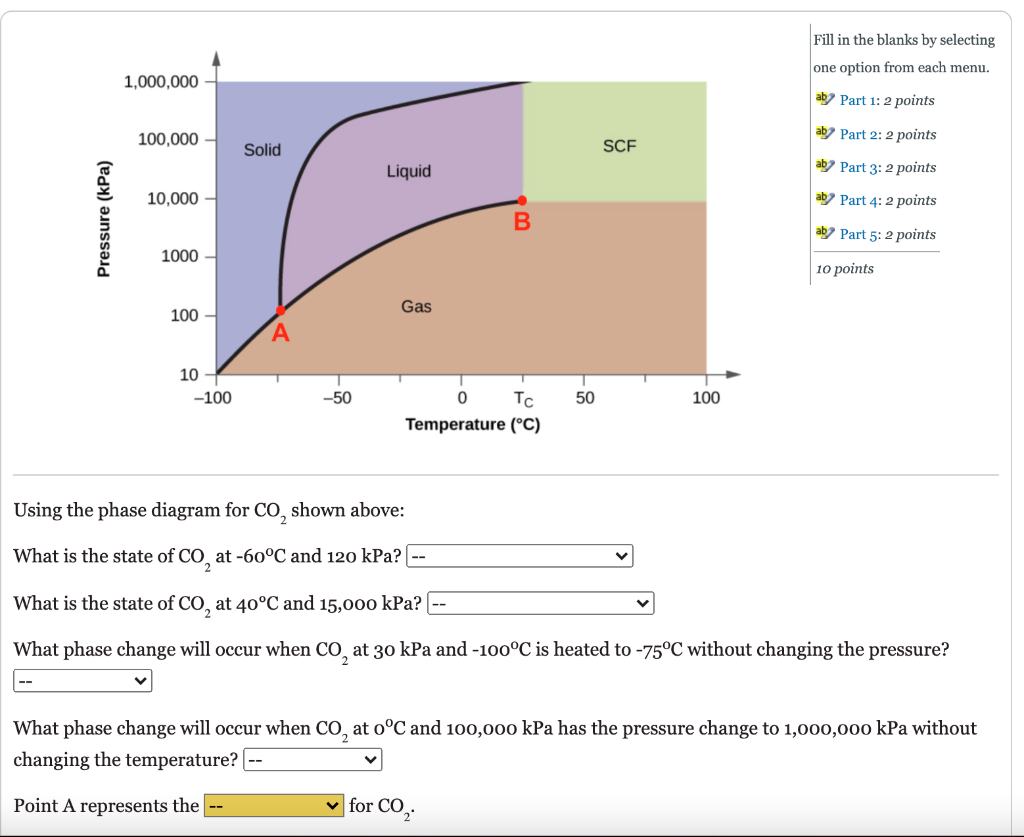
Fill in the blanks by selecting one option from each menu. 1,000,000 ab Part 1: 2 points 100,000 aby Part 2: 2 points Solid SCF Liquid ab Part 3:2 points 10,000 ab Part 4: 2 points Pressure (kPa) B. aby Part 5:2 points 1000 10 points 100 Gas 10 -100 -50 1 50 100 TC Temperature (C) Using the phase diagram for CO shown above: What is the state of CO, at -60C and 120 kPa? -- What is the state of Co, at 40C and 15,000 kPa?| What phase change will occur when CO at 30 kPa and -100C is heated to -75C without changing the pressure? What phase change will occur when Co, at oC and 100,000 kPa has the pressure change to 1,000,000 kPa without changing the temperature? -- Point A represents the for CO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


