Question: fill the enthalpy table Aliquid stream containing 49.5 mole% benzene and the balance toluene at 30.0C is fed to a continuous single-stage evaporator at a
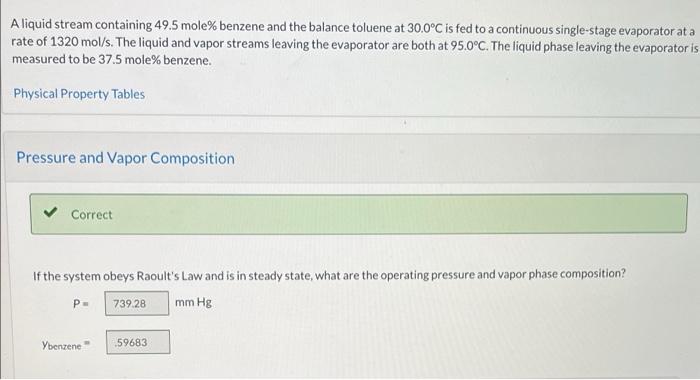
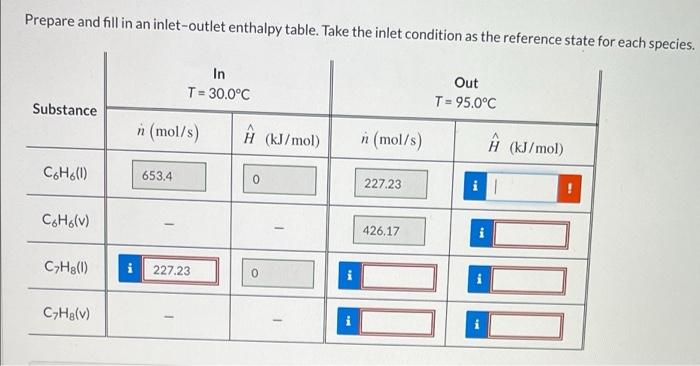
Aliquid stream containing 49.5 mole% benzene and the balance toluene at 30.0C is fed to a continuous single-stage evaporator at a rate of 1320 mol/s. The liquid and vapor streams leaving the evaporator are both at 95.0C. The liquid phase leaving the evaporator is measured to be 37.5 mole% benzene. Physical Property Tables Pressure and Vapor Composition Correct If the system obeys Raoult's Law and is in steady state, what are the operating pressure and vapor phase composition? P- 739 28 mm Hg Ybenzene .59683 Prepare and fill in an inlet-outlet enthalpy table. Take the inlet condition as the reference state for each species. In T = 30.0C Out T = 95.0C Substance n (mol/s) (kJ/mol) n (mol/s) (kJ/mol) C6H6(0 653.4 0 227.23 1 C.H.(V) 426,17 CyH80) 227.23 0 CyHelv)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


