Question: FINAL PROPER SIGNIFICAT FIGURES III. ANSWER AS DIRECTED. Give the detailed solution to the following problems. Express your final answer in the proper number of
FINAL PROPER SIGNIFICAT FIGURES
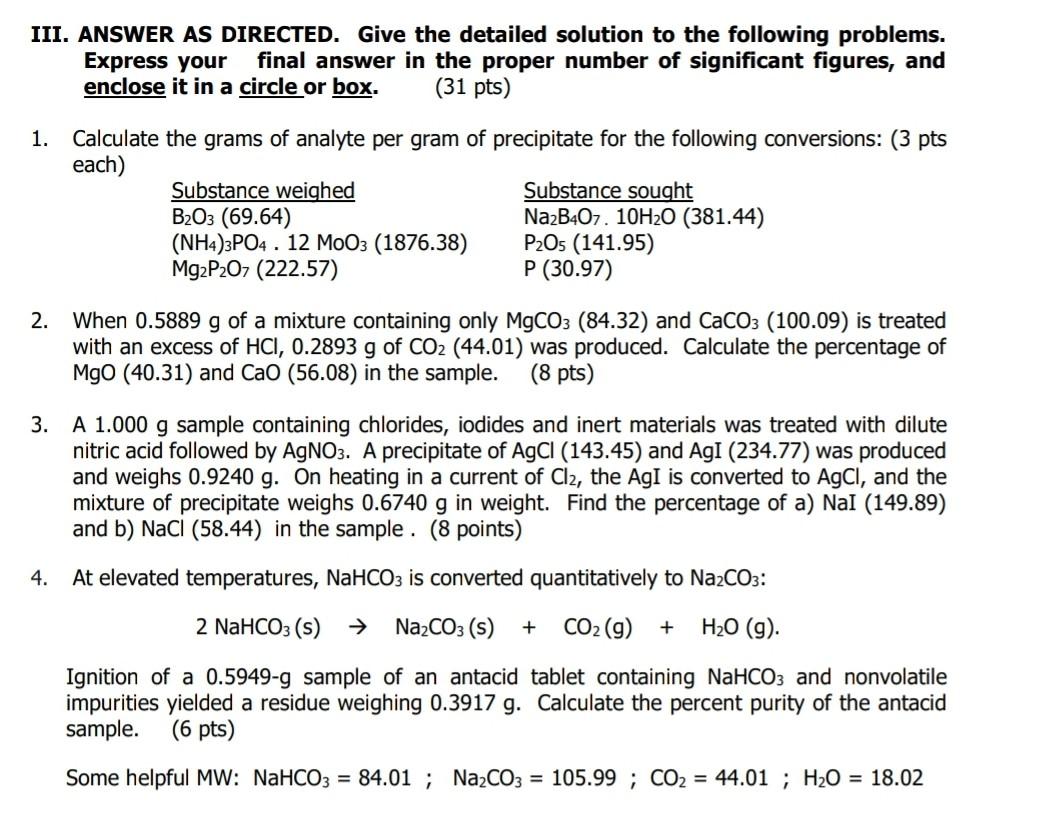
III. ANSWER AS DIRECTED. Give the detailed solution to the following problems. Express your final answer in the proper number of significant figures, and enclose it in a circle or box. (31 pts) 1. Calculate the grams of analyte per gram of precipitate for the following conversions: (3 pts each) Substance weighed Substance sought B2O3 (69.64) Na2B4O7. 10H20 (381.44) (NH4)3PO4 . 12 MoO3 (1876.38) P2O5 (141.95) Mg2P2O7 (222.57) P (30.97) 2. When 0.5889 g of a mixture containing only MgCO3 (84.32) and CaCO3 (100.09) is treated with an excess of HCI, 0.2893 g of CO2 (44.01) was produced. Calculate the percentage of MgO (40.31) and CaO (56.08) in the sample. (8 pts) 3. A 1.000 g sample containing chlorides, iodides and inert materials was treated with dilute nitric acid followed by AgNO3. A precipitate of AgCl (143.45) and AgI (234.77) was produced and weighs 0.9240 g. On heating in a current of Cl2, the AgI is converted to AgCl, and the mixture of precipitate weighs 0.6740 g in weight. Find the percentage of a) Nal (149.89) and b) NaCl (58.44) in the sample. (8 points) 4. At elevated temperatures, NaHCO3 is converted quantitatively to Na2CO3: + 2 NaHCO3 (5) Na2CO3 (s) CO2(g) + H20 (9) Ignition of a 0.5949-g sample of an antacid tablet containing NaHCO3 and nonvolatile impurities yielded a residue weighing 0.3917 g. Calculate the percent purity of the antacid sample. (6 pts) Some helpful MW: NaHCO3 = 84.01 ; Na2CO3 = 105.99 ; CO2 = 44.01 ; H2O = 18.02
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


