Question: Finally consider a Galvanic electrochemical cell operating at 24C that is constructed from a 2.20105MACl2 (aq) solution with a A (s) electrode coupled to a
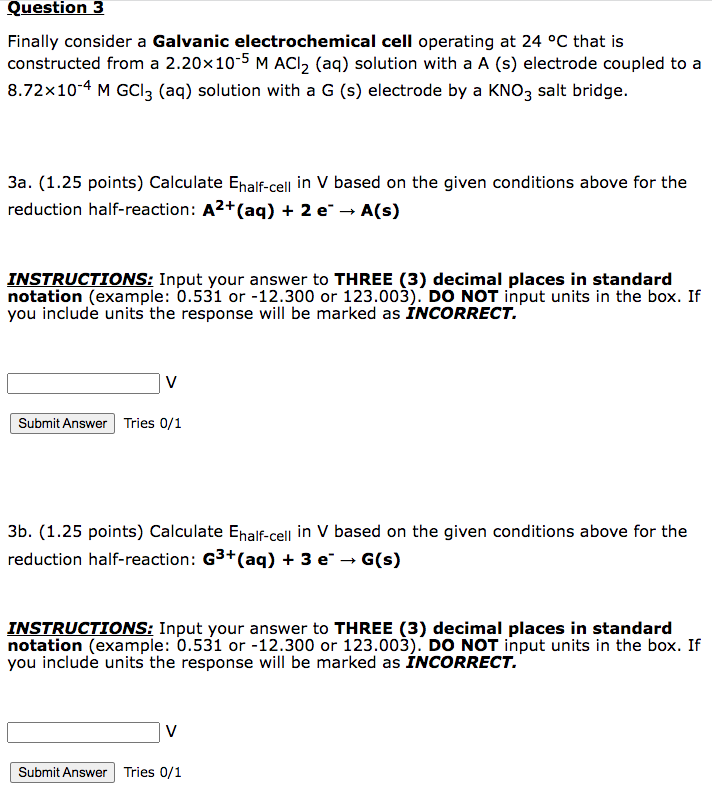
Finally consider a Galvanic electrochemical cell operating at 24C that is constructed from a 2.20105MACl2 (aq) solution with a A (s) electrode coupled to a 8.72104MGCl3(aq) solution with a G (s) electrode by a KNO3 salt bridge. 3a. (1.25 points) Calculate Ehalf-cell in V based on the given conditions above for the reduction half-reaction: A2(aq)+2eA(s) INSTRUCTIONS: Input your answer to THREE (3) decimal places in standard notation (example: 0.531 or -12.300 or 123.003 ). DO NOT input units in the box. If you include units the response will be marked as INCORRECT. V Tries 0/1 3b. (1.25 points) Calculate Ehalf-cell in V based on the given conditions above for the reduction half-reaction: G3((aq)+3eG(s) INSTRUCTIONS: Input your answer to THREE (3) decimal places in standard notation (example: 0.531 or -12.300 or 123.003 ). DO NOT input units in the box. If you include units the response will be marked as INCORRECT. V Tries 0/1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


