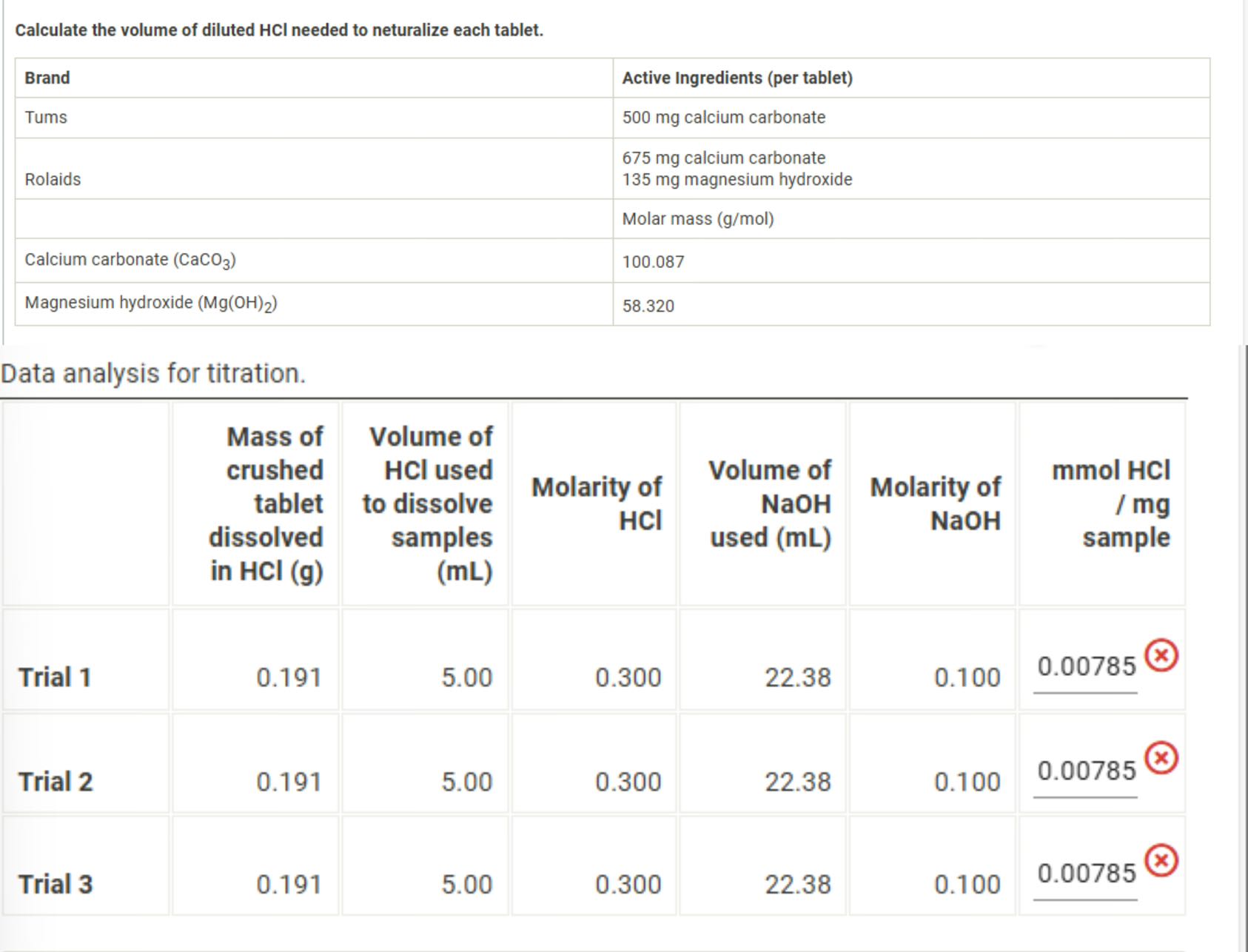
Question: find the correct mmol HCl / mg sample,The difference between the millimoles of HCl used to dissolve the sample and the millimoles of NaOH required
find the correct mmol HCl / mg sample,The difference between the millimoles of HCl used to dissolve the sample and the millimoles of NaOH required to titrate the residual acid, when divided by the antacid sample mass in milligrams, is the number you are seeking.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


