Question: find the ph calculation for a solution with a ph of 8.93 and a concentration of 0.10M please put the chemical equation on there as
find the ph calculation for a solution with a ph of 8.93 and a concentration of 0.10M
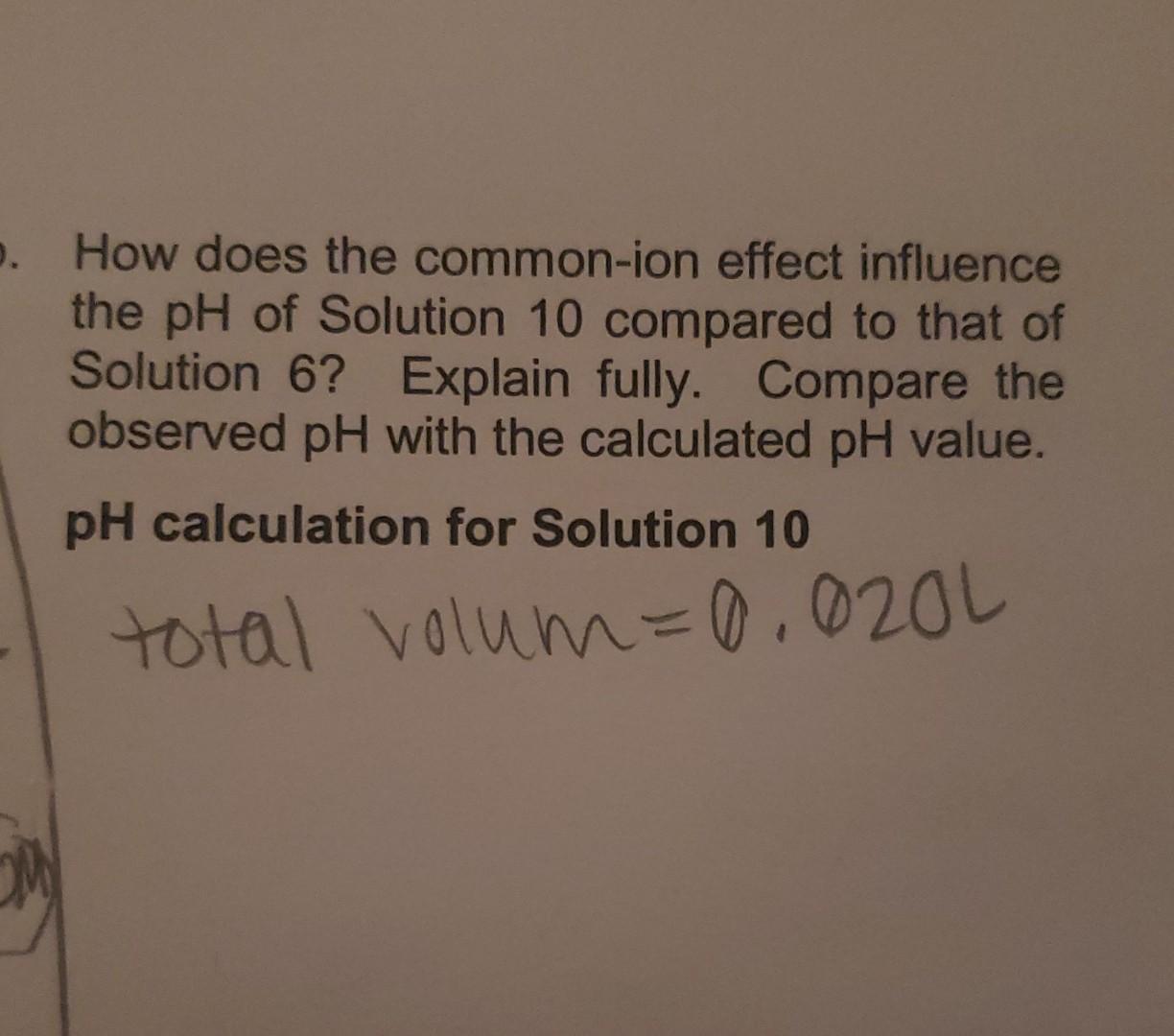
please put the chemical equation on there as well thank you
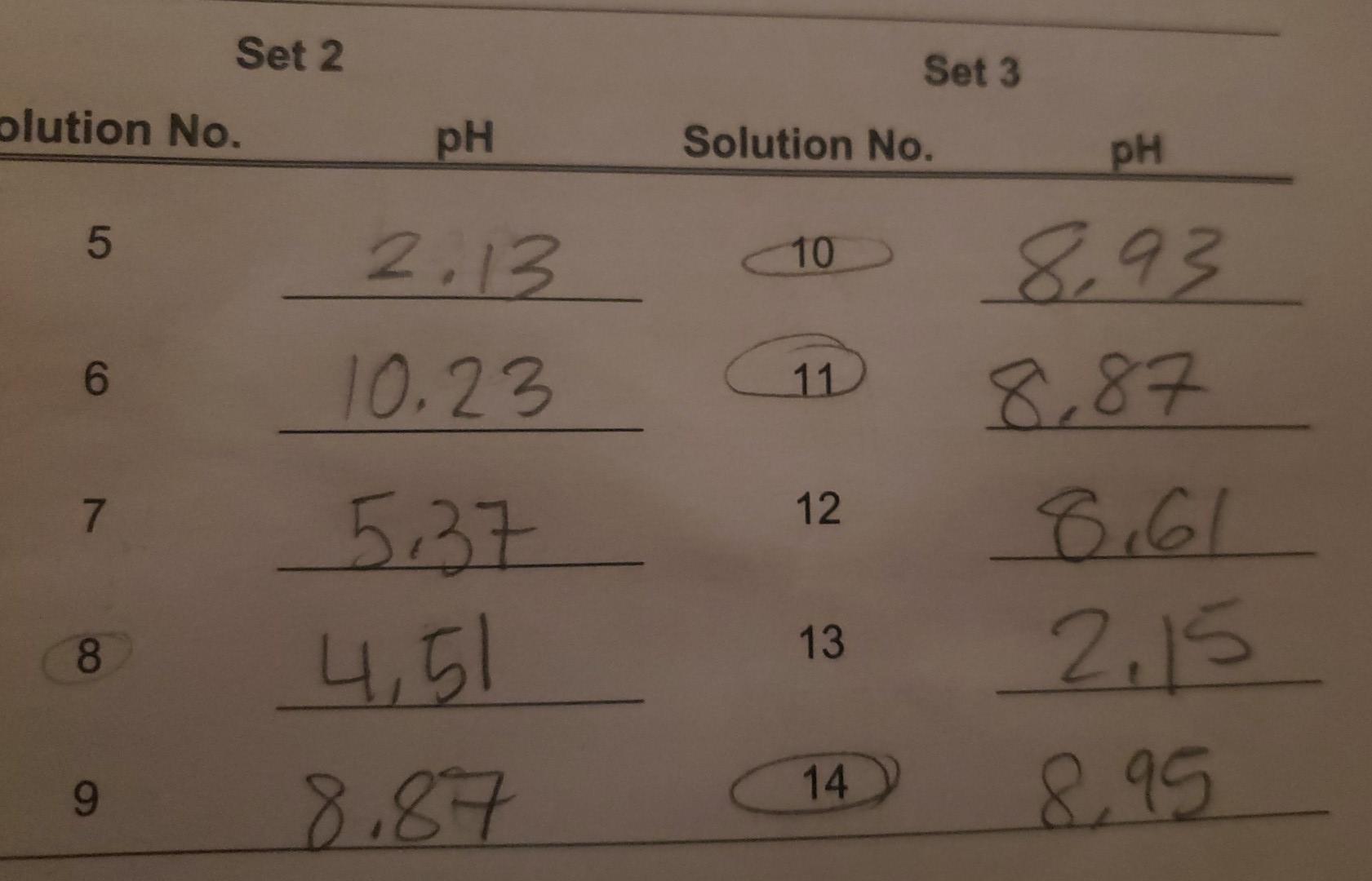
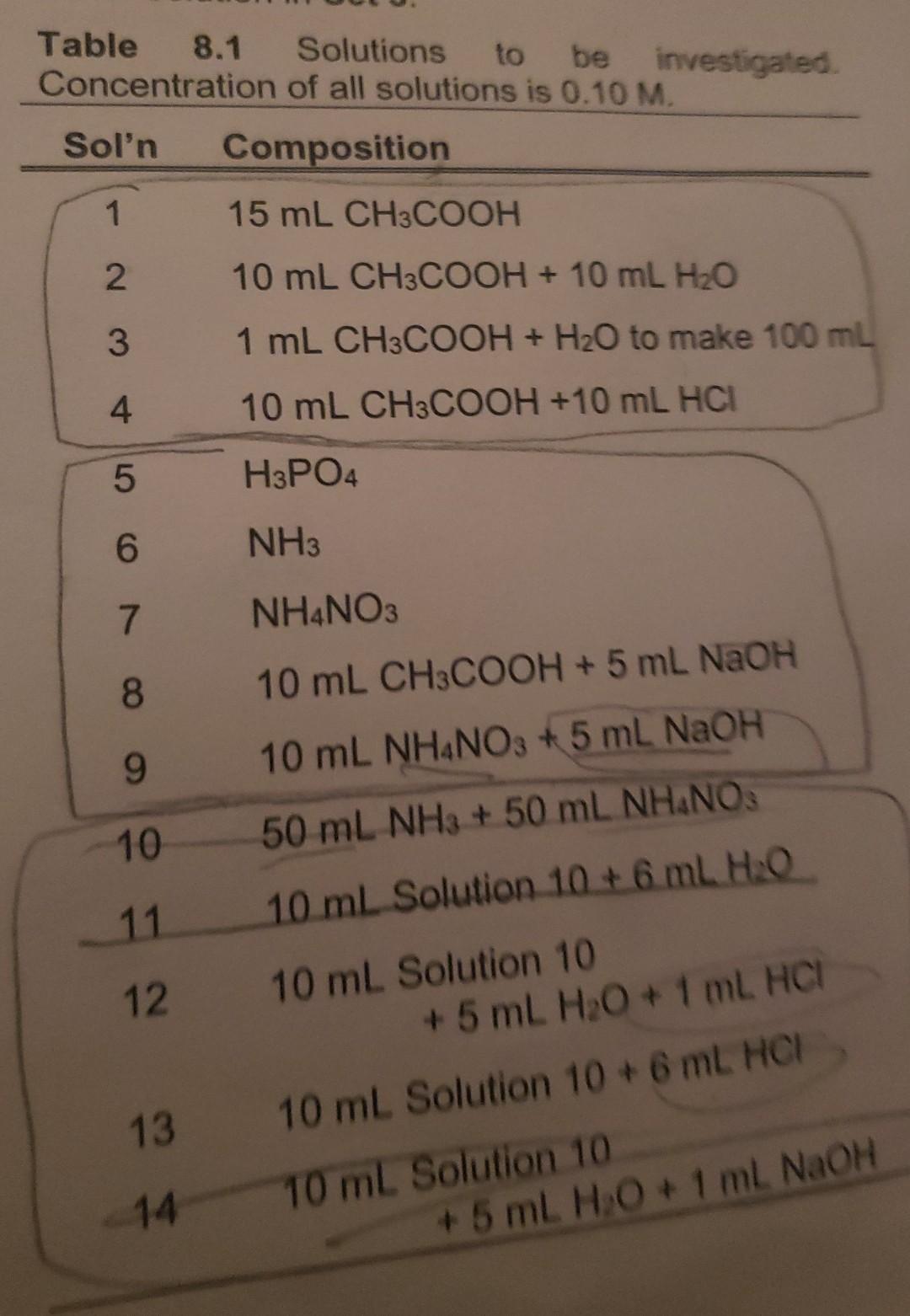
. How does the common-ion effect influence the pH of Solution 10 compared to that of Solution 6? Explain fully. Compare the observed pH with the calculated pH value. pH calculation for Solution 10 total volum=0.020L Set 2 Set 3 olution No. ph Solution No. pH 5 2.13 10 6 10.23 11 8,93 8,87 80 2.15 7 12. 8. 13 5,37 , 5 8,87 9 14 8.95 Table 8.1 Solutions to be investigated. Concentration of all solutions is 0.10 M. Sol'n Composition 1 15 mL CH3COOH 2. 10 mL CH3COOH + 10 mL H2O 3 1 mL CH3COOH + H2O to make 100 ml 4 10 mL CH3COOH +10 mL HCI 5 H3PO4 6 NH3 7 NH4NO3 8. 10 mL CH3COOH + 5 mL NAOH 9 10 mL NH4NO3 + 5 mL NAOH 10 50 mL NH3 + 50 mL NHANOS 11 10 mL Solution 10 + 6 mL HO 12 10 mL Solution 10 + 5 mL H2O + 1 ml HCI 13 10 ml Solution 10+ 6 mL HCI 14 10 ml Solution 10 + 5 mL H2O + 1 ml NaOH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


