Question: () Find the temperature increase that would be expected if all the energy of plastic deformation only were to be converted into heat instead of

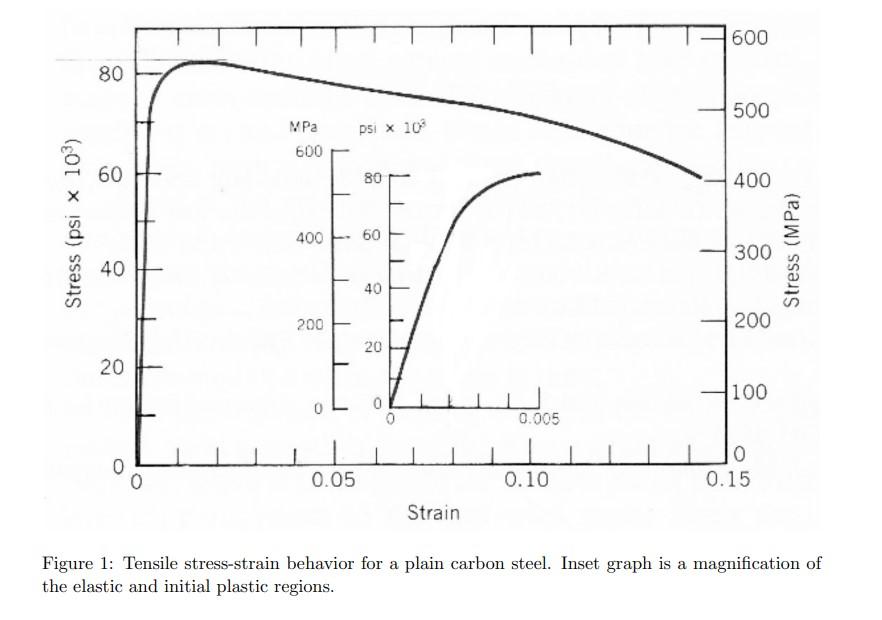
() Find the temperature increase that would be expected if all the energy of plastic deformation only were to be converted into heat instead of being stored as dislocations or dissipated into the surrounding environment. The specific heat of carbon steel alloys is 486 J/kg-K and the density is 7.85 g/cm' or 7850 kg/m3. (k) Find the increase in dislocation density that would result if all the energy of plastic defor- mation only were to be stored as dislocations instead of being dissipated as heat. (Note that in actual tests both effects, heating and increased dislocation density, are present.) To solve this problem, you'll need to find a number for energy per length of dislocation, calculated as Q = G62, where Q is energy per length, G is the shear modulus, b is the magnitude of the Burgers vector, corresponding to a jump between two atoms in a slip system. 600 80 500 MPa psi x 10 10%) 600 60 80 400 Stress (psix 400 60 300 Stress (MPa) 40 40 200 200 20 20 100 0 0.005 1 0 0.15 0.05 0.10 Strain Figure 1: Tensile stress-strain behavior for a plain carbon steel. Inset graph is a magnification of the elastic and initial plastic regions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


