Question: Find thermodynamic quantities for a process that doesn't fall into any previously discussed category. P(10 Pa) 3.00 2.00 LL 1.00- V(m) 0.100 0.200 0.300
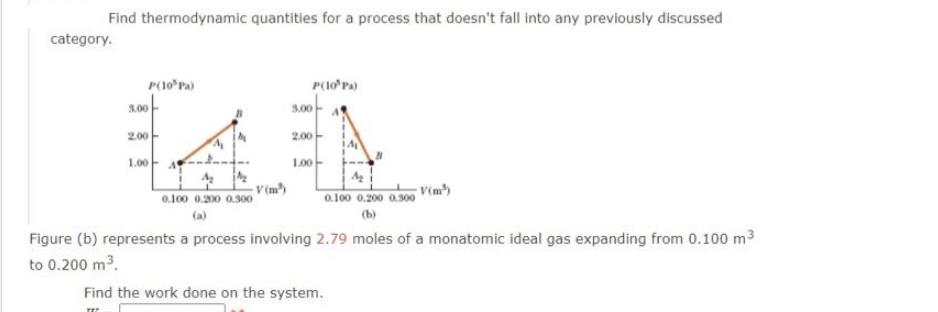

Find thermodynamic quantities for a process that doesn't fall into any previously discussed category. P(10 Pa) 3.00 2.00 LL 1.00- V(m) 0.100 0.200 0.300 P(10 Pa) 3.00 2.00 1.00 0.100 0.200 0.300 (b) Figure (b) represents a process involving 2.79 moles of a monatomic ideal gas expanding from 0.100 m to 0.200 m. Find the work done on the system. Find the change in the internal energy of the system. AU = J Find the thermal energy transferred in the process.
Step by Step Solution
3.41 Rating (148 Votes )
There are 3 Steps involved in it
a Calculating the work done on system b Work done on ... View full answer

Get step-by-step solutions from verified subject matter experts


