Question: First item. Just copy the entry I made below for the disposal of mL of 100 ppm As as As(NO 3 ) 3 that is
- First item. Just copy the entry I made below for the disposal of mL of 100 ppm As as As(NO3)3 that is approximately 0.1 M in nitric acid HNO3. This is two ingredients in one solution, right. Note below how I handle two ingredient mixtures.
- Ingredient 1 is on the first line, and no volume is specified. Instead, I say "with below".
- Ingredient 2 is on the second line, and a volume is specified. (100 mL)
- Second item: On your form, include as well an entry for the addition, to the same container, of 155 mL of 5% HCl solution that also contains 250 ppm CuCl2 and 0.10 M HCl.
- Third item: On your form, include as well an entry for the addition, to the same container, a solution from an experiment in which you have digested one multi-vitamin tablet in 30 mL of 35% HCl. You then diluted the whole to 500 mL and wish to dispose of all of it as waste. Calculate the new concentrations and make an entry in the waste log that reflects your best estimates and description of the contents. Since a vitamin tablet comprises a mixture of safe organic chemicals, metals and some inert ingredients, it is sufficient to indicate that it is a "dissolved vitamin tablet" for the technicians downstream to properly handle it.
- Use one waste form to log both components listed above (the idea is that they were put into the same waste container).
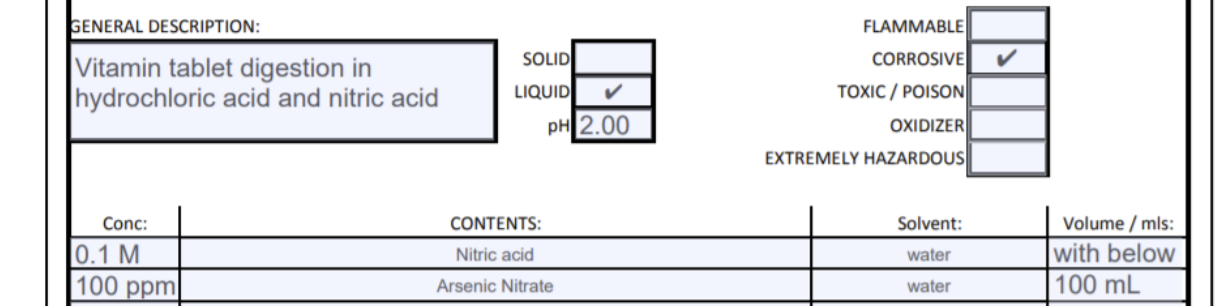
GENERAL DESCRIPTION: FLAMMABLE SOLID CORROSIVE Vitamin tablet digestion in hydrochloric acid and nitric acid LIQUID TOXIC / POISON pH 2.00 OXIDIZER EXTREMELY HAZARDOUS Solvent: Conc: 10.1 M 100 ppm CONTENTS: Nitric acid water Volume/mls: with below 100 mL Arsenic Nitrate water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


