Question: First - order reversible reaction, A r R , C R 0 C A 0 = M , kinetics approximated or fitted by - r
Firstorder reversible reaction, kinetics approximated or fitted by with an observed equilibrium conversion any constant
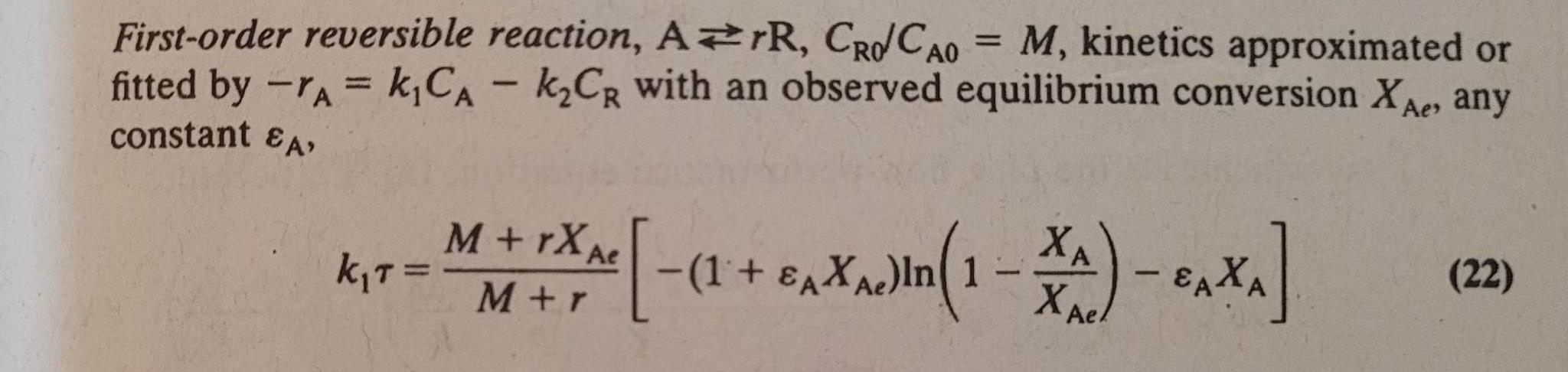
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


