Question: (five marks) Use the following information to answer the next question. Methanol is known to be the simplest alcohol, and is used as an antifreeze
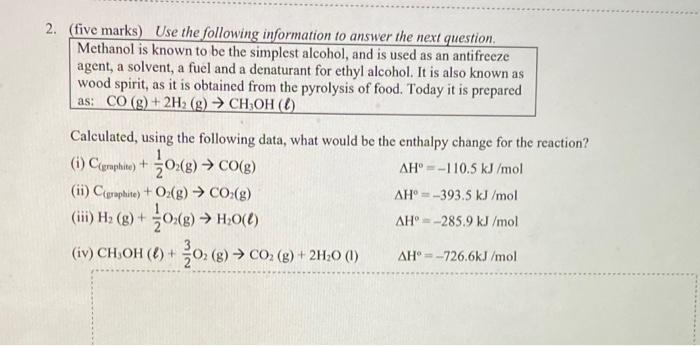
(five marks) Use the following information to answer the next question. Methanol is known to be the simplest alcohol, and is used as an antifreeze agent, a solvent, a fuel and a denaturant for ethyl alcohol. It is also known as wood spirit, as it is obtained from the pyrolysis of food. Today it is prepared as: CO(g)+2H2(g)CH3OH() Calculated, using the following data, what would be the enthalpy change for the reaction? (i) C(graphite)+21O2(g)CO(g) H=110.5kJ/mol (ii) C(eraplite)+O2(g)CO2(g) H=393.5kJ/mol (iii) H2(g)+21O2(g)H2O() H=285.9kJ/mol (iv) CH3OH()+33O2(g)CO2(g)+2H2O (I) H=726.6kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


