Question: For 210.0mL of a buffer solution that is 0.200M in HCHO2 and 0.315M in KCHO2, calculate the initial pH and the final pH after adding
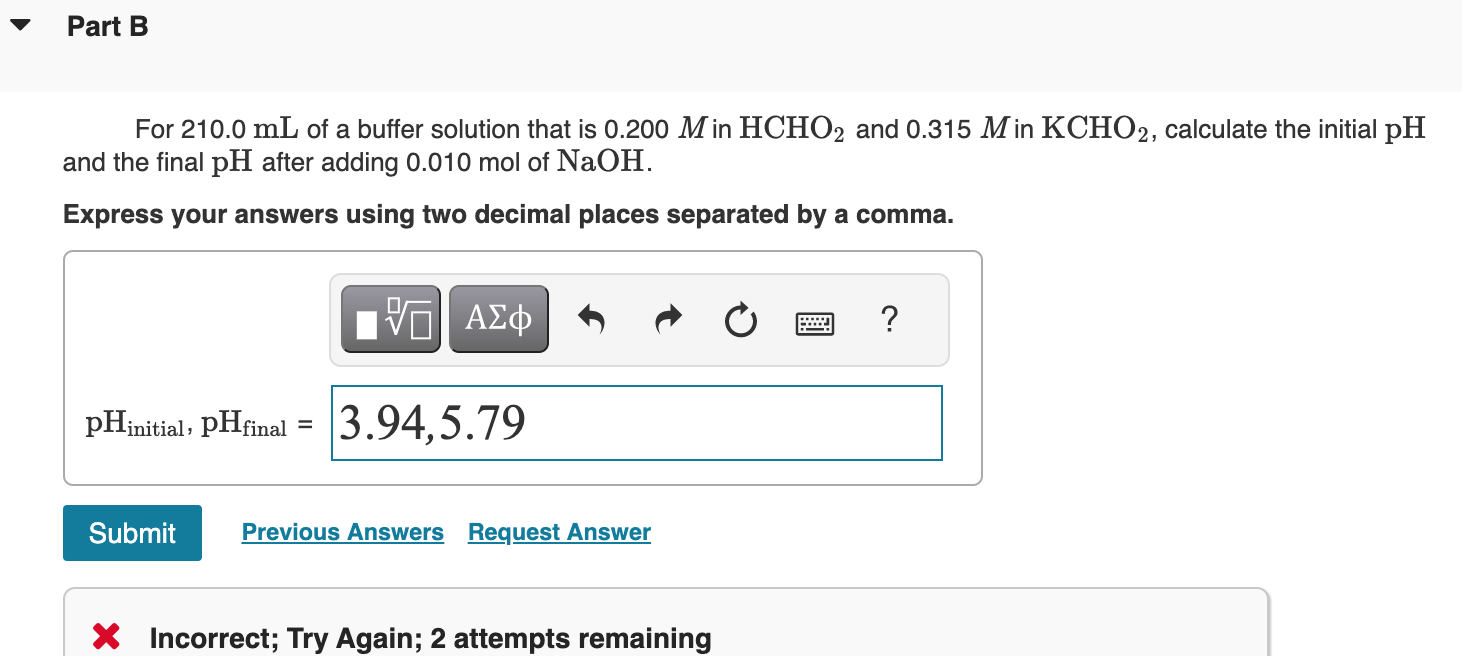
For 210.0mL of a buffer solution that is 0.200M in HCHO2 and 0.315M in KCHO2, calculate the initial pH and the final pH after adding 0.010mol of NaOH. Express your answers using two decimal places separated by a comma. pHinitial,pHfinal= X Incorrect; Try Again; 2 attempts remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


