Question: For a 10C rise in temperature, the rate constant doubles for reaction I and triples for reaction II. If both reactions have the same
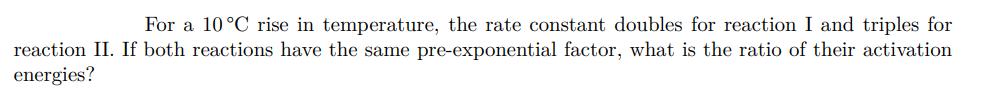
For a 10C rise in temperature, the rate constant doubles for reaction I and triples for reaction II. If both reactions have the same pre-exponential factor, what is the ratio of their activation energies?
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The detailed ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


