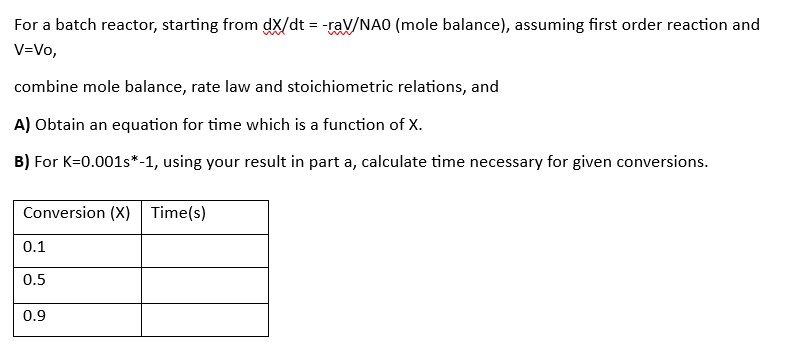
Question: For a batch reactor, starting from d x d t = - r a V N A O ( mole balance ) , assuming first
For a batch reactor, starting from mole balance assuming first order reaction and
combine mole balance, rate law and stoichiometric relations, and
A Obtain an equation for time which is a function of
B For using your result in part a calculate time necessary for given conversions.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


