Question: For (a), Cp value pls use data from table B2 and heat of formation method. Tq, will rate the answer. Calcium oxide (Cao) is usually
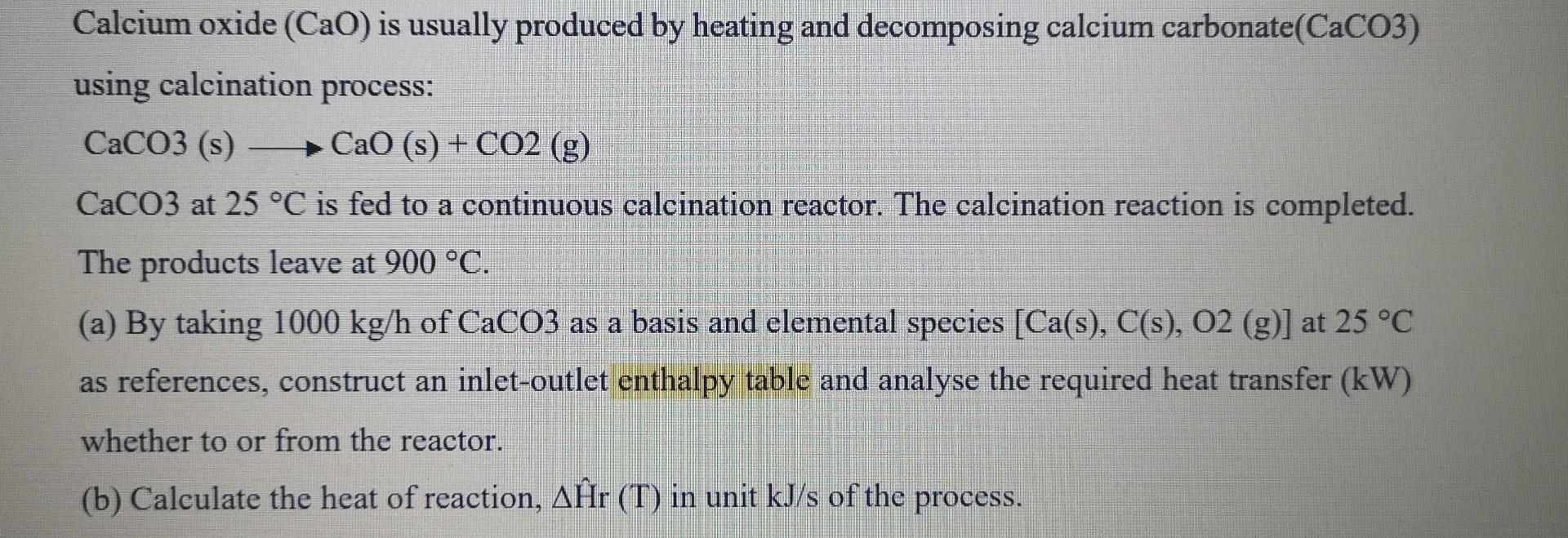
For (a), Cp value pls use data from table B2 and heat of formation method. Tq, will rate the answer.
Calcium oxide (Cao) is usually produced by heating and decomposing calcium carbonate(CaCO3) using calcination process: CaCO3 (s) CaO (s)+ CO2 (g) CaCO3 at 25 C is fed to a continuous calcination reactor. The calcination reaction is completed. The products leave at 900 C. (a) By taking 1000 kg/h of CaCO3 as a basis and elemental species [Ca(s), C(s), 02 (9)] at 25 C as references, construct an inlet-outlet enthalpy table and analyse the required heat transfer (kW) whether to or from the reactor. (b) Calculate the heat of reaction, Ar (T) in unit kJ/s of the process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


