Question: For a silver (Ag) - copper (Cu) solution at 1400K, the variation of solution enthalpy (HM) and entropy (SM) with molar ratio is given as
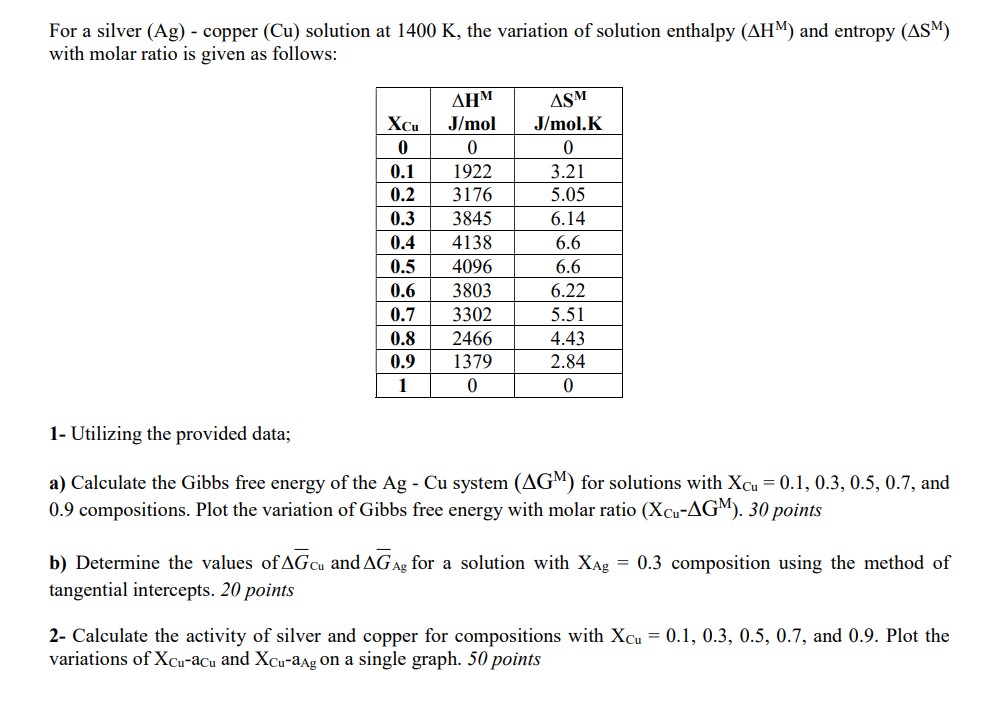
For a silver (Ag) - copper (Cu) solution at 1400K, the variation of solution enthalpy (HM) and entropy (SM) with molar ratio is given as follows: 1- Utilizing the provided data; a) Calculate the Gibbs free energy of the AgCu system (GM) for solutions with XCu=0.1,0.3,0.5,0.7, and 0.9 compositions. Plot the variation of Gibbs free energy with molar ratio (XCuGM).30 points b) Determine the values of GCu and GAg for a solution with XAg=0.3 composition using the method of tangential intercepts. 20 points 2- Calculate the activity of silver and copper for compositions with XCu=0.1,0.3,0.5,0.7, and 0.9. Plot the variations of XCuaCu and XCuaAg on a single graph. 50 points
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


