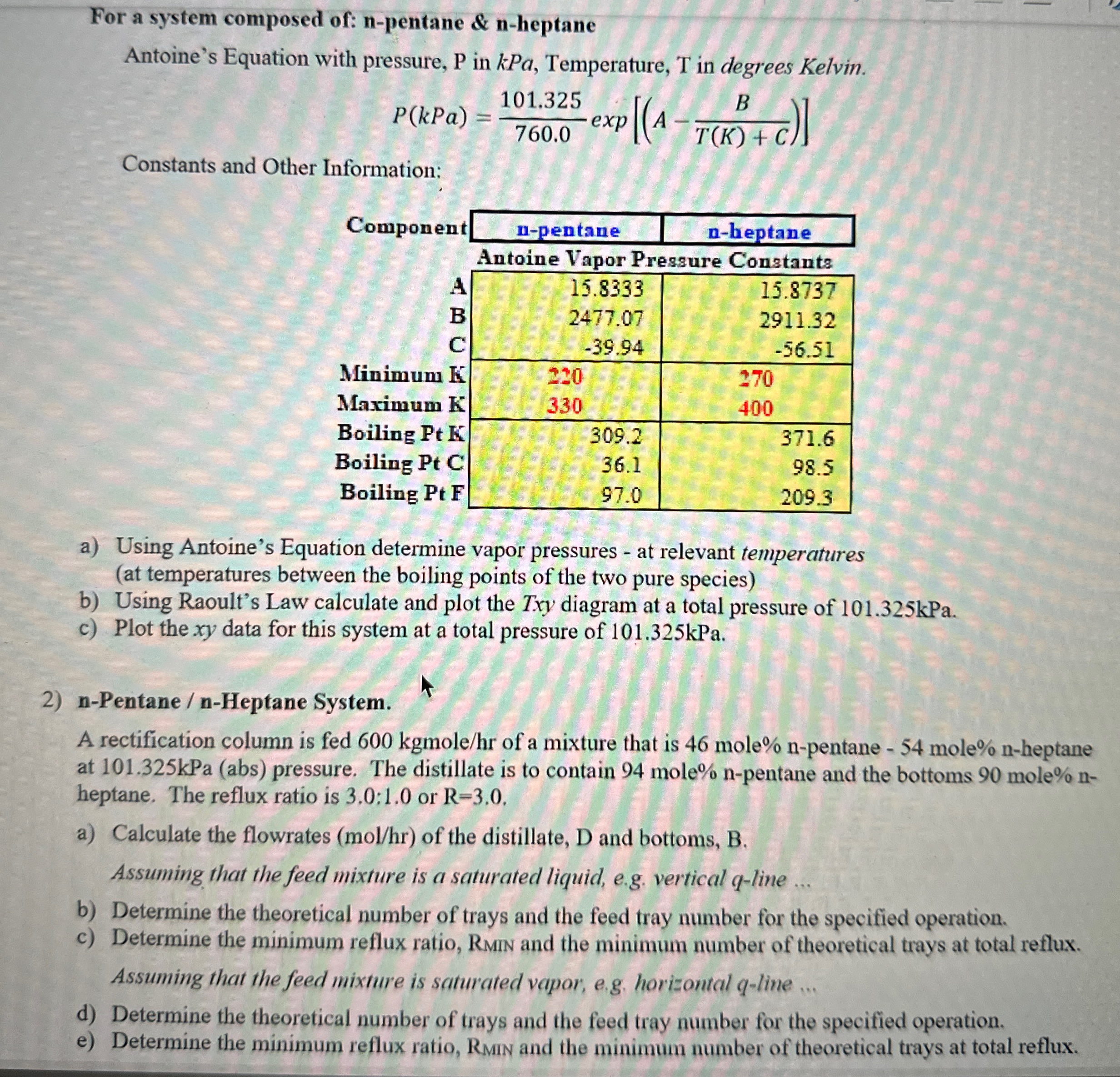
Question: For a system composed of: n - pentane & n - heptane Antoine's Equation with pressure, P in kPa, Temperature, T in degrees Kelvin. P
For a system composed of: npentane & nheptane
Antoine's Equation with pressure, in kPa, Temperature, in degrees Kelvin.
exp
Constants and Other Information:
tableComponentnpentane,nheptaneAntoine Vapor Pressure Constants,BCMinimum KMaximum KBoiling Pt KBoiling Pt CBoiling Pt F
a Using Antoine's Equation determine vapor pressures at relevant temperatures at temperatures between the boiling points of the two pure species
b Using Raoult's Law calculate and plot the diagram at a total pressure of kPa.
c Plot the data for this system at a total pressure of kPa.
nPentane nHeptane System.
A rectification column is fed kgmol of a mixture that is mole npentane mole nheptane at kPa abs pressure. The distillate is to contain mole npentane and the bottoms mole nheptane. The reflux ratio is : or
a Calculate the flowrates of the distillate, and bottoms,
Assuming that the feed mixture is a saturated liquid, eg vertical line
b Determine the theoretical number of trays and the feed tray number for the specified operation.
c Determine the minimum reflux ratio, Rmin and the minimum number of theoretical trays at total reflux. Assuming that the feed mixture is saturated vapor, eg horizontal line
d Determine the theoretical number of trays and the feed tray number for the specified operation.
e Determine the minimum reflux ratio, RMIN and the minimum number of theoretical trays at total reflux.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


