Question: For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1
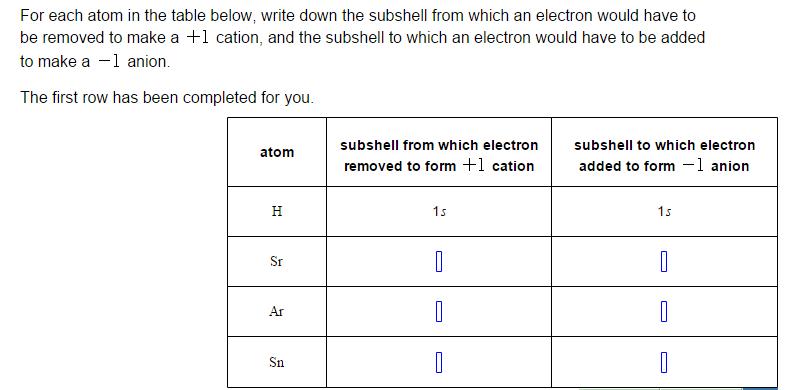
For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you. subshell from which electron subshell to which electron atom removed to form +1 cation added to form -1 anion H 1s 1s Sr Ar Sn
Step by Step Solution
3.36 Rating (143 Votes )
There are 3 Steps involved in it
Atom Subshell from wh... View full answer

Get step-by-step solutions from verified subject matter experts


