Question: For each carbocation: a. Classify accordingly. b. Is this stable enough to react with benzene? If so, write YES. If not, then the carbocation must
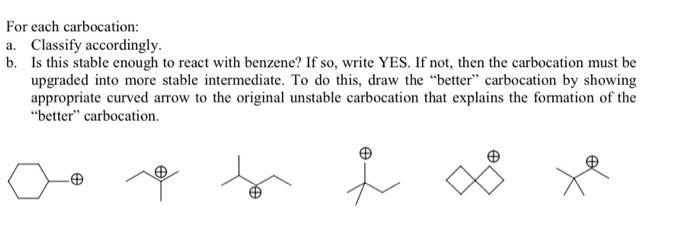
For each carbocation: a. Classify accordingly. b. Is this stable enough to react with benzene? If so, write YES. If not, then the carbocation must be upgraded into more stable intermediate. To do this, draw the "better" carbocation by showing appropriate curved arrow to the original unstable carbocation that explains the formation of the "better" carbocation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


