Question: For each cylinder shown below, determine the (a) uncertainty, (b) volume of liquid in cylinder, and (a) number of significant figures in your measurement. The
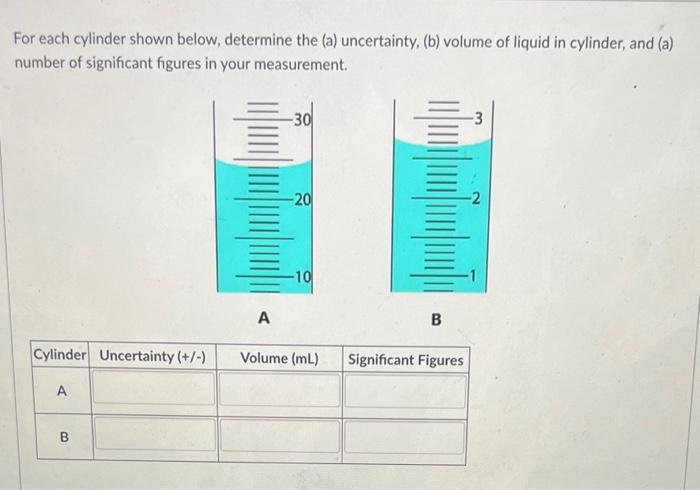

For each cylinder shown below, determine the (a) uncertainty, (b) volume of liquid in cylinder, and (a) number of significant figures in your measurement. The mass of an object was determined as 24.8563g using an analytical balance. What would be the expected mass of this object on each of the balances listed below. (When entering answers below, do not include units) a) Centigram balance: b) Milligram balance: c) Decigram balance
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


