Question: = For each solution, determine the p-values for each ion indicated. A solution that is 0.357M in NaCl and 0.103M in NH4Cl. pNa= pCl= pNH4=
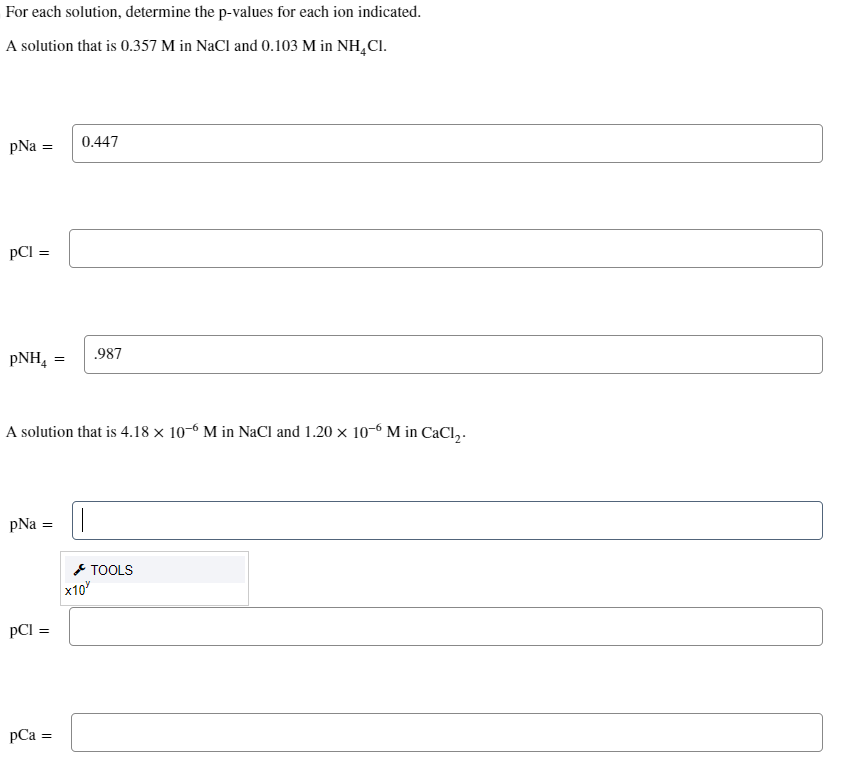
= For each solution, determine the p-values for each ion indicated. A solution that is 0.357M in NaCl and 0.103M in NH4Cl. pNa= pCl= pNH4= A solution that is 4.18106M in NaCl and 1.20106M in CaCl2. pNa= pCl= pCa=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


