Question: for for these 4 examples and one question. please explain the steps and what I have to do to solve them. Thank you! Calculate Kp

for for these 4 examples and one question. please explain the steps and what I have to do to solve them. Thank you!
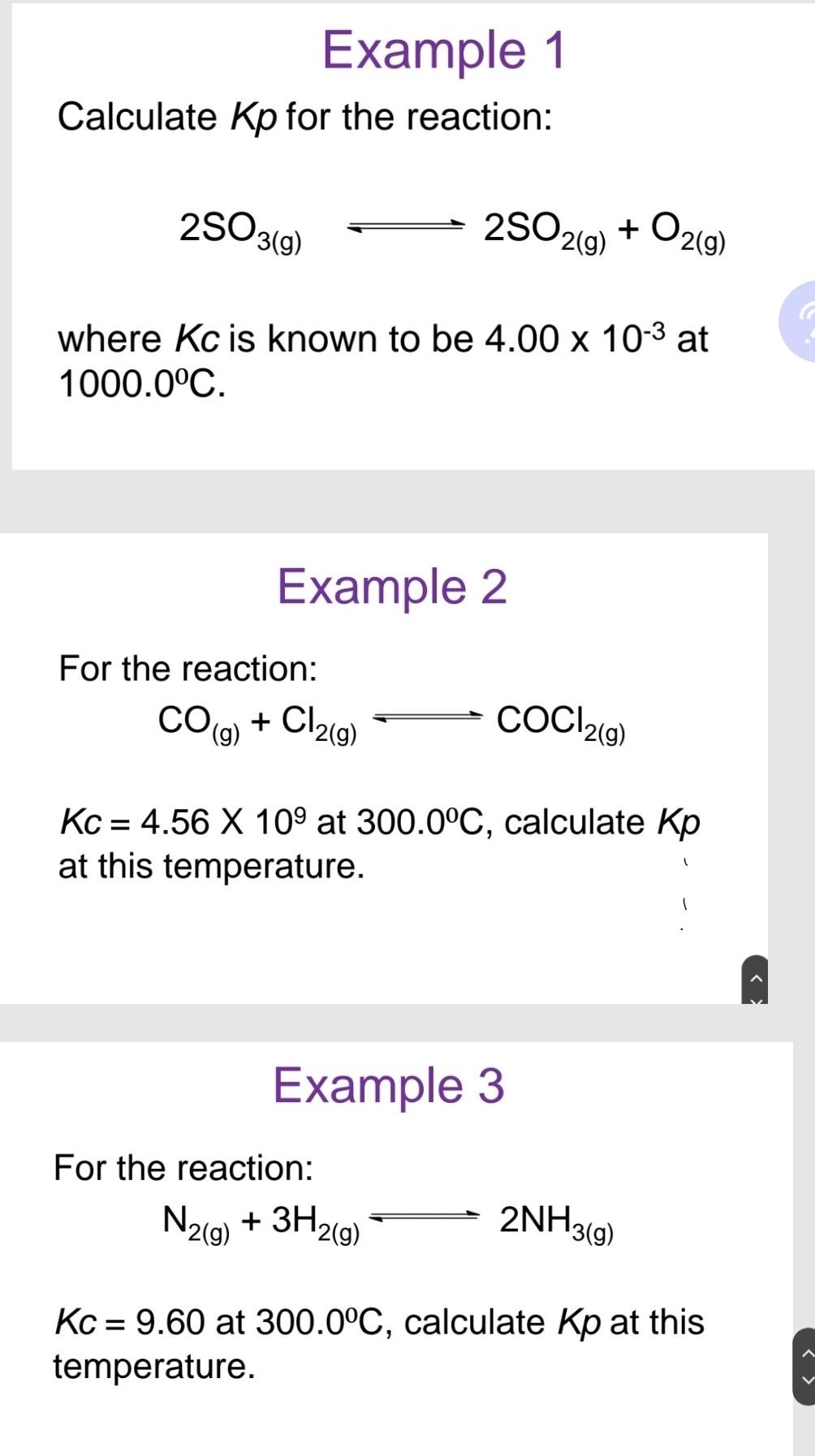
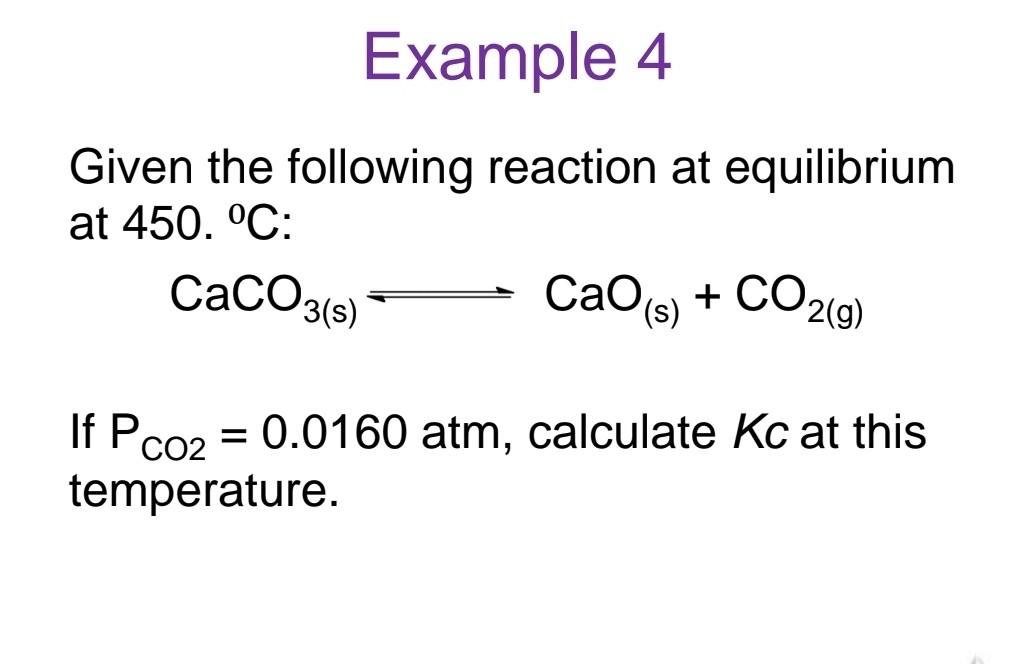
Calculate Kp for the reaction: 2SO3(g)2SO2(g)+O2(g) where Kc is known to be 4.00103 at 1000.0C. Example 2 For the reaction: CO(g)+Cl2(g)COCl2(g) Kc=4.56109 at 300.0C, calculate Kp at this temperature. Example 3 For the reaction: N2(g)+3H2(g)2NH3(g) Kc=9.60 at 300.0C, calculate Kp at this temperature. Given the following reaction at equilibrium at 450. C : CaCO3(s)CaO(s)+CO2(g) If PCO2=0.0160atm, calculate Kc at this temperature. A mixture of 0.10MNO,0.050MH2 and 0.10M H2O is placed in a closed vessel. The following equilibrium is established: 2NO(g)+2H2(g)N2(g)+2H2O(g) At equilibrium [NO]=0.062M a. Find the equilibrium concentrations of H2,N2 and H2O b. Find Kc
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


