Question: For items 2 7 - 2 8 : Below is data collected for the analysis of C a in a supplement tablet using atomic absorption
For items :
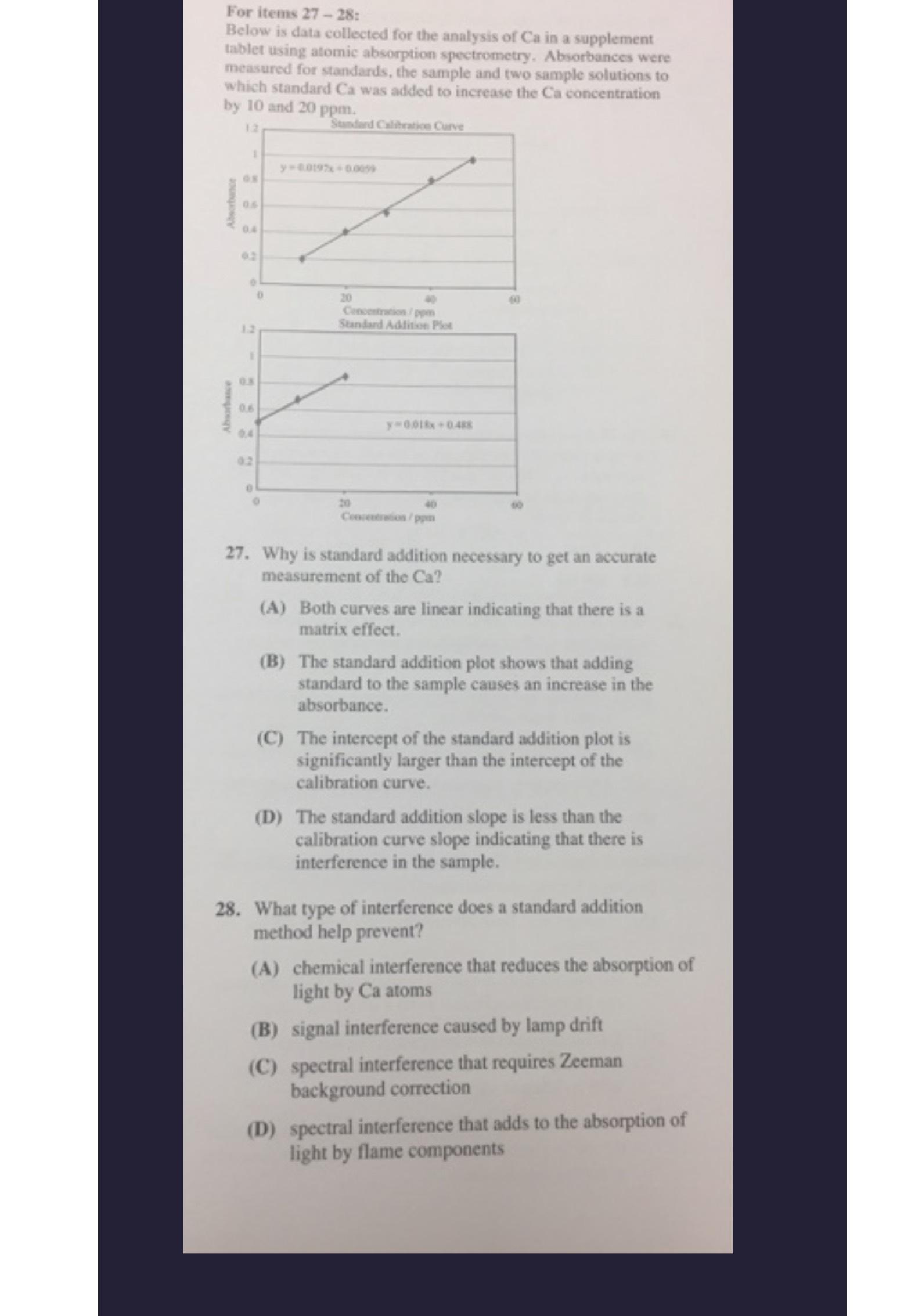
Below is data collected for the analysis of in a supplement tablet using atomic absorption spectrometry. Absorbances were measured for standards, the sample and two sample solutions to which standard Ca was added to increase the Ca concentration
Why is standard addition necessary to get an accurate measurement of the
A Both eurves are linear indicating that there is a matrix effect.
B The standard addition plot shows that adding standard to the sample causes an increase in the absorbance
C The intercept of the standard addition plot is significantly larger than the intercept of the calibration curve.
D The standard addition slope is less than the calibration curve slope indicating that there is interference in the sample.
What type of interference does a standard addition method help prevent?
A chemical interference that reduces the absorption of light by atoms
B signal interference caused by lamp drift
C spectral interference that requires Zeeman background correction
D spectral interference that adds to the absorption of light by flame components
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


