Question: For neutral molecules, which statements about covalent Lewis structures are true? Drag each item to the appropriate bin. Hydrogen atoms are often the central
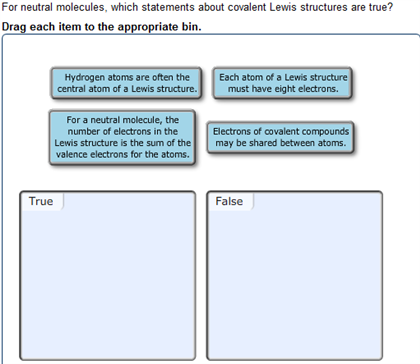
For neutral molecules, which statements about covalent Lewis structures are true? Drag each item to the appropriate bin. Hydrogen atoms are often the central atom of a Lewis structure. For a neutral molecule, the number of electrons in the Lewis structure is the sum of the valence electrons for the atoms. True Each atom of a Lewis structure must have eight electrons. Electrons of covalent compounds may be shared between atoms. False
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Q For neutral molecules which staments about Covalent Lewis structures are tr... View full answer

Get step-by-step solutions from verified subject matter experts


