Question: For q1. Please include range that goes from 1.5-2.5cm e.g using volume and uncertainty for q.3 please include percentage uncertainty for the beaker and cylinder
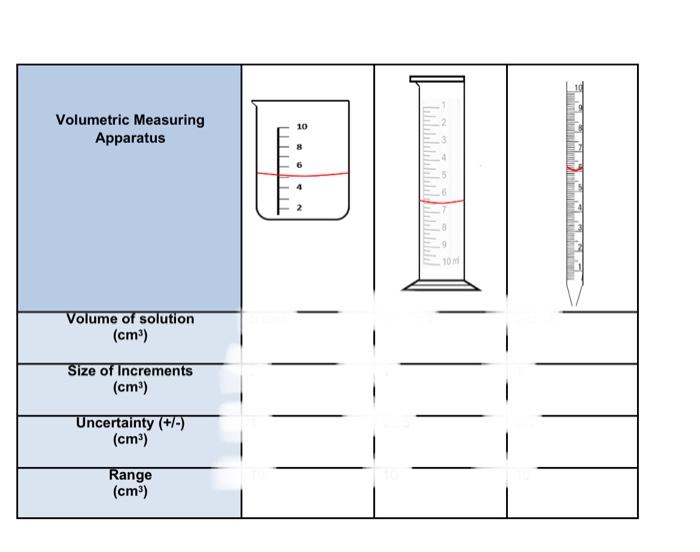
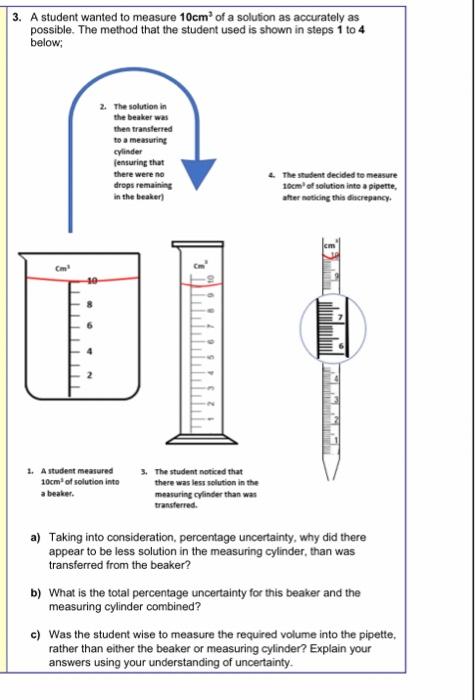
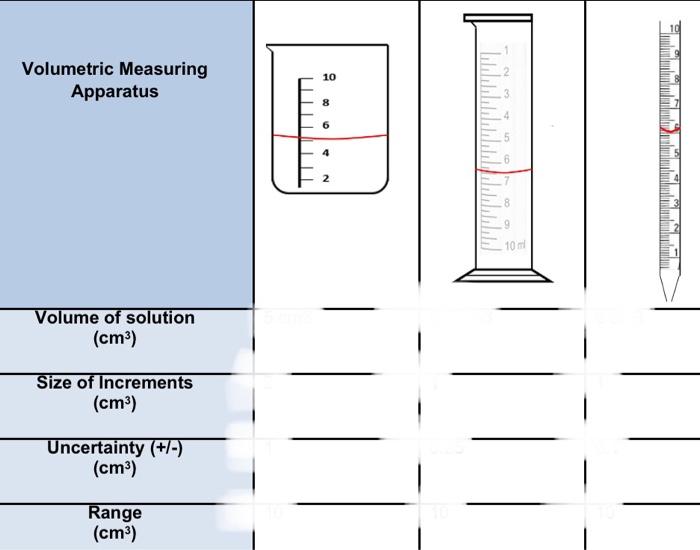
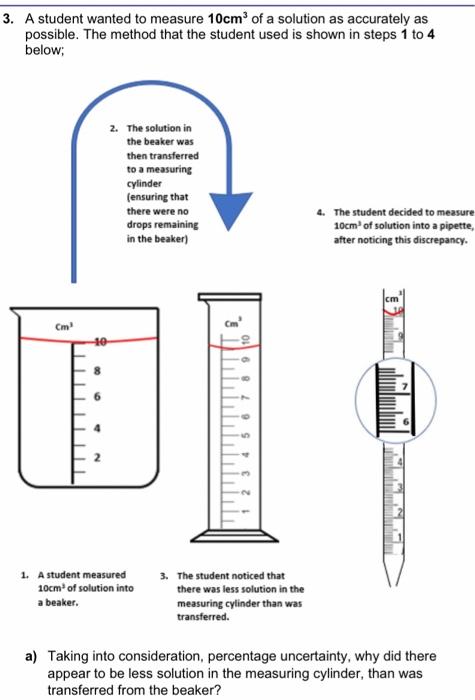

3. A student wanted to measure 10cm2 of a solution as accurately as possible. The method that the student used is shown in steps 1 to 4 below; 2. The solution in the beaker was then transterred to a measuring cylinder fensuring that there were no 4. The gtudent decided to measure drops remaining 10cm3 of solution inte a pipette, in the beaker) after motiding this dincripancy. 1. A student measured 3. The student noticed that 10cm2 of solution inte there was less selution in the a beaker. measuring cylinder than was transferred. a) Taking into consideration, percentage uncertainty, why did there appear to be less solution in the measuring cylinder, than was transferred from the beaker? b) What is the total percentage uncertainty for this beaker and the measuring cylinder combined? c) Was the student wise to measure the required volume into the pipette, rather than either the beaker or measuring cylinder? Explain your answers using your understanding of uncertainty. Volumetric Measuring Apparatus Size of Increments (cm3) Uncertainty (+/-) (cm3) Range (cm3) 3. A student wanted to measure 10cm3 of a solution as accurately as possible. The method that the student used is shown in steps 1 to 4 below; 4. The student decided to measure 10cm3 of solution into a pipette, after noticing this discrepancy. 1. A student measured 3. The student noticed that 10cm3 of solution into there was less solution in the measuring cylinder than was transferred. a) Taking into consideration, percentage uncertainty, why did there appear to be less solution in the measuring cylinder, than was transferred from the beaker? a) Taking into consideration, percentage uncertainty, why did there appear to be less solution in the measuring cylinder, than was transferred from the beaker? b) What is the total percentage uncertainty for this beaker and the measuring cylinder combined? c) Was the student wise to measure the required volume into the pipette rather than either the beaker or measuring cylinder? Explain your answers using your understanding of uncertainty
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


