Question: For questions 2, 3, & 5... What equations would I use to solve these problems? I am confused on how I am supposed to get

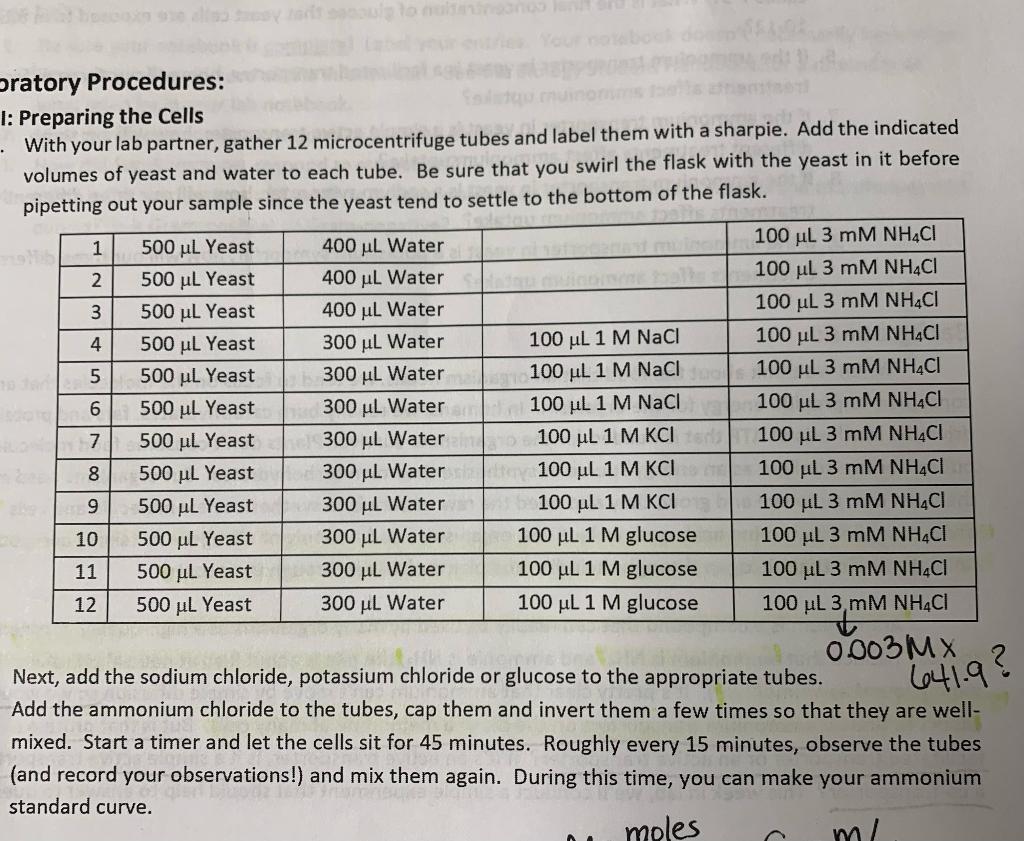
For questions 2, 3, & 5... What equations would I use to solve these problems? I am confused on how I am supposed to get these answers. I provided the table to reference, there are 12 samples that are all in separate microcentrifuge tubes so I am unsure what final amount and final concentration the yeast would be exposed to. If anyone can help it would be greatly appreciated!!
There is NO additional prelab information. This is why I am so confused on the questions because it does not provide how to solve for them.
Answer the following pre-lab questions in your lab notebook: 1. Why are we pretty confident that ammonium can't move by simple diffusion at a neutral pH? *2. What is the final concentration of ammonium chloride that the yeast cells will be exposed to? Provide your answer using units of uM. *3. What amount of ammonium will the cells have access to? Provide your answer using units of nmoles. * What volumes of the 3 mM NHCl solution will we need to pipet for the standard curve? - 5. What is the final concentration of sodium chloride that the yeast cells are exposed to in tubes 4-6? What is the final concentration of potassium chloride that yeast cells are exposed to in tubes 7-9? What is the final concentration of glucose that yeast cells are exposed to in tubes 10-12? in vont is a facilitated transporter, how will our three different Sratory Procedures: 1: Preparing the Cells With your lab partner, gather 12 microcentrifuge tubes and label them with a sharpie. Add the indicated volumes of yeast and water to each tube. Be sure that you swirl the flask with the yeast in it before pipetting out your sample since the yeast tend to settle to the bottom of the flask. 1 500 ML Yeast 400 ML Water 100 ul 3 mM NHACI 2 500 uL Yeast 400 uL Water 100 ML 3 MM NH4Cl 3 500 ul Yeast 400 ul Water 100 uL 3 mM NH4CI 4 500 ul Yeast 300 ul Water 100 uL 1 M NaCl 100 ul 3 mM NHACI 5 500 ul Yeast 300 ML Water 100 ML 1 M NaCl 100 uL 3 mM NH4Cl 6 500 uL Yeast 300 ul Water an 100 uL1M NaCl 100 ul 3 mM NHACI | 7 500 ul Yeast 300 ul Water 100 uL 1 M KCI 100 uL 3 mM NHACI 8 500 ul Yeast 300 ul Water de 100 ul 1 M KCI 100 uL 3 MM NHACI 9 500 ul Yeast 300 ul Water 100 ML 1 M KCI 100 ul 3 mM NHACI 10 500 ul Yeast 300 ul Water 100 uL 1 M glucose 100 uL 3 MM NHACI 11 500 ul Yeast 300 ML Water 100 ML 1 M glucose 100 uL 3 mM NHACI 12 500 uL Yeast 300 uL Water 100 ul 1 M glucose 100 ul 3 mM NH4Cl 3.MN 0.003 MX Next, add the sodium chloride, potassium chloride or glucose to the appropriate tubes. Add the ammonium chloride to the tubes, cap them and invert them a few times so that they are well- mixed. Start a timer and let the cells sit for 45 minutes. Roughly every 15 minutes, observe the tubes (and record your observations!) and mix them again. During this time, you can make your ammonium standard curve. moles m/ 6641.9? 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


