Question: For reference, use the following electronegativity values: C 2.5 N 3.0 03.5 1. Consider the three canonical structures of the fulminate ion (CNO-): [:C=N=0:] or
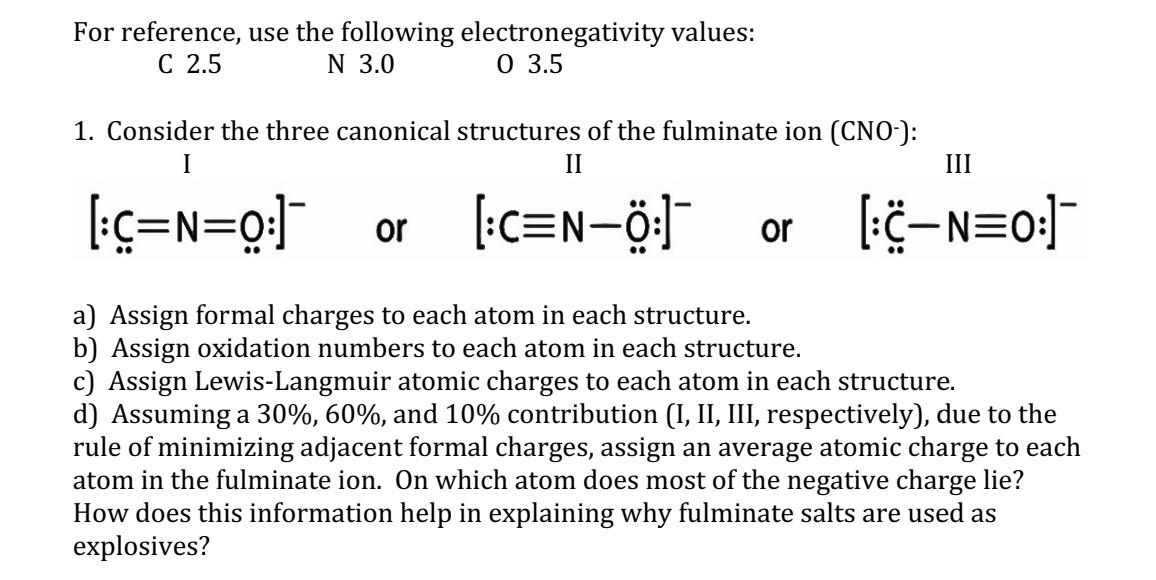
For reference, use the following electronegativity values: C 2.5 N 3.0 03.5 1. Consider the three canonical structures of the fulminate ion (CNO-): [:C=N=0:] or [:CN0.0:] or [:CN0:] a) Assign formal charges to each atom in each structure. b) Assign oxidation numbers to each atom in each structure. c) Assign Lewis-Langmuir atomic charges to each atom in each structure. d) Assuming a 30\%, 60\%, and 10\% contribution (I, II, III, respectively), due to the rule of minimizing adjacent formal charges, assign an average atomic charge to each atom in the fulminate ion. On which atom does most of the negative charge lie? How does this information help in explaining why fulminate salts are used as explosives
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


